2.2 Rh(III) porphyrin ammonia complex
After a routine preparation and purification of 118 in the manner described in section 2.1, a product was obtained with an 1H NMR spectrum which in addition to the peaks expected for 118 also displayed a singlet peak at 10.23 ppm, characteristic of the meso resonance of an undesired porphyrinic byproduct. Addition of an excess of pyridine to this product yielded largely the expected Rh-pyridine complex, as judged by the NMR spectrum (section 2.4.2), and a decrease in the intensity of the peak at 10.23 ppm, with this peak disappearing altogether after evaporation of volatile matter and drying in vacuo. This implied that the likely cause of the impurity was a volatile ligand becoming coordinated to the rhodium centre during synthesis or purification, and that addition of pyridine competed for the coordination site displacing this ligand which was finally removed when the sample was dried.
A re-examination of the NMR spectrum acquired with a wide sweep width revealed a singlet peak at -5.77 ppm substantiating the hypothesis of a coordinated ligand. After further experimentation the source of this ligand was found to be the silica gel used for chromatography. This batch had been received from a new supplier, and had been used for the first time instead of that supplied by Merck which was used in the original preparation of 118. Stirring a solution of 118 in chloroform with a large quantity of the silica converted it cleanly to 119, which was identified as an ammonia complex on the basis of the 3H singlet at -5.77 ppm, and from the results of nitrogen microanalysis. Confirmation was obtained by direct synthesis of 119 by exposure of 118 to ammonia; the product of this reaction had identical spectroscopic properties to that obtained by silica treatment. Evidently traces of ammonia were adsorbed to this silica and this became complexed to rhodium during the chromatographic step. Following this discovery all chromatography of rhodium porphyrin was carried out on Merck silica gel which avoided this problem.
There have been few examples of spectroscopically or crystallographically characterized metalloporphyrin ammonia complexes described in the literature. It has been known for some time that ammonia will complex to peptide linked heme groups237 although only more recently have complexes of ammonia with simple Fe(II),238 Fe(III),239 Co(III),240 Os(II),241 and Ru(II)242 porphyrins been prepared.
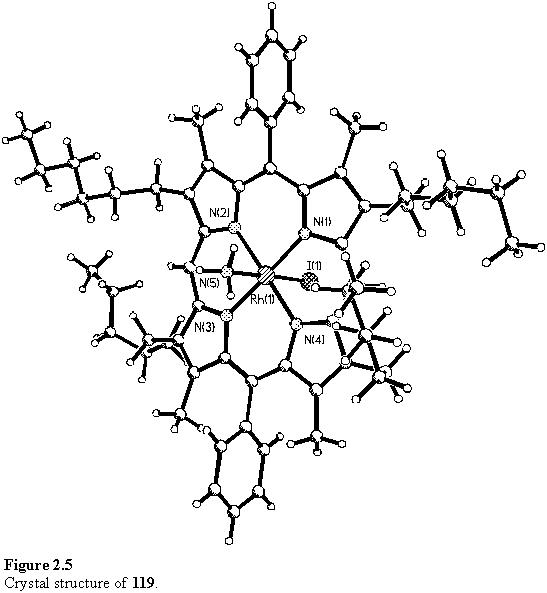
As was often observed with these rhodium porphyrin coordination compounds, attempts to obtain a MALDI mass spectrum of 119 failed to generate a molecular ion peak. The ammonia ligand was lost and the only meaningful peaks observed were at m/z 1084 [M-NH3]+ and 957 [M-NH3-I]+. Weak peaks in the IR spectrum at 3381 and 3274 cm-1 are tentatively assigned to the NH3 group. An X-ray crystallographic analysis of 119 was performed and the structure is shown in figure 2.5. The porphyrin is slightly distorted from planarity, and the bond length to the axial nitrogen atom is 2.113(5) Å.