2.4 Rh porphyrin coordination to bidentate ligands
As a first step towards the creation of larger structures based on Rh porphyrin coordination chemistry it was decided to examine the reaction of 118 with bidentate ligands. For this goal, tight and specific binding is preferential as it ensures complete assembly of the desired structure. A slow exchange of free and bound ligands on the NMR chemical shift time-scale is also advantageous as this leads to sharp spectra in which the proportions of each species may be estimated by integration of resonances.
Requirements for templating ligands245 are that they should bind reactants in a geometry that accelerates product formation and/or slows the formation of alternative product structures. A means of removal of the template after reaction completion is an asset. For Zn porphyrin oligomers templated by multidentate pyridine ligands this can be achieved by acidification which protonates the ligand and porphyrins thus releasing the bound template. However such a procedure was found to be ineffective when applied to a templated rhodium porphyrin dimer.246
2.4.1 Rh(III) porphyrin 4,4'-bipyridine complex
4,4'-Bipyridine was chosen as a starting point as it is one of the simplest commercially available bidentate pyridyl ligands and there is a wealth of information available on its use as a template for porphyrin oligomerization reactions.246,247
The bipyridine complex, 121, was prepared in 75% yield by addition of a THF solution of 118 to an excess of 4,4'-bipyridine in THF, followed by column chromatography. In contrast a literature preparation220 of the analogous (OEP)Rh(III)Cl complex used 0.5 eq of bipyridine in refluxing DCM. In the present case, refluxing was unnecessary.
121 was not the expected product under the reaction conditions employed, as the large excess of bipyridine was expected to favour a 1:1 porphyrin:bipyridine stoichiometry. This result either indicated a large cooperative binding of the porphyrin by 4,4'-bipyridine, or initial formation of the expected 1:1 complex followed by conversion to 121 during the isolation procedure.
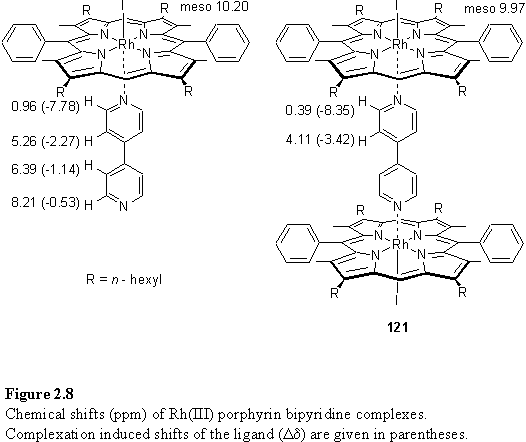
The 1H NMR spectrum of 121 shows highly shielded and sharp bipyridine Ha and Hb resonances at 0.39 and 4.11 ppm respectively and a slightly shifted meso resonance at 9.97 ppm. Comparable NMR spectra of bipyridine complexes were reported by Thomas220 and Kadish101 for octaethyl and tetraphenyl Rh(III) porphyrins, and by Anderson102 and Darling248 for Ru(II) porphyrins.
To determine the initially formed product distribution obtained on reaction of 118 with 4,4'-bipyridine a solution of 118·MeOH was titrated with bipyridine in CDCl3 and the product composition was monitored by 1H NMR spectroscopy. On addition of up to 0.5 eq of bipyridine the only product observed was 121, and displaced MeOH which appeared as a doublet at the expected chemical shift of 3.49 ppm.249 Further addition of bipyridine resulted in appearance of a new set of resonances attributed to the 1:1 complex, with a meso resonance at 10.20 ppm. The chemical shifts and complexation induced shifts (Dd) for these species are shown in figure 2.8. The Dd values of 121 are almost precisely the sum of the Dd observed in the 1:1 complex. Possible evidence of an electronic effect on the ligand on coordination to the porphyrin is an increase in the coupling constant between the a and b pyridyl protons from 5 to 7 Hz.
As all species are in slow exchange the equilibrium constant, K, for the reaction of equation 1 can be determined by integration of the appropriate peaks.
 Equation 1
Equation 1
This equilibrium constant is equal to the ratio of the equilibrium constants for the second (K2) and first (K1) porphyrin complexations by the bidentate ligand:
![]()
K=K1/K2 Equation 2
The cooperativity coefficient,250 a, can be defined as a = K/4 where the factor of 4 takes into account the statistics of binding. Two independent determinations gave an average value of the cooperativity coefficient for this system as a = 1.9 indicating the porphyrins bind largely non-cooperatively to each end of the ligand (a = 1 for non cooperative binding and a < 1 for cooperative binding), a result which parallels that obtained for Ru(II)CO mesoporphyrin(II)-dimethyl ester.251
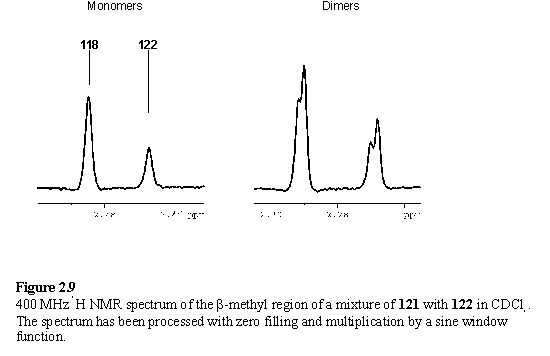
The possibility of isolation of mixed complexes of the type P1-bipyridine-P2 is precluded by the exchange of the porphyrin units. Addition of ester substituted porphyrin, 122, to a solution of 121 yielded a mixture of dimers and 118 as evidenced by a pair of closely spaced meso resonances at 10.31 ppm corresponding to 118 and 122, and two resolved peaks at 9.98 and 9.96 ppm arising from dimers. All possible combinations of dimers and monomers were present in the mixture as demonstrated by the observation of 6 b-methyl resonances (figure 2.9).
In a preliminary experiment to judge the exchange rates the 1H NMR spectrum of a sample of 118 and excess 4,4'-bipyridine in C2D2Cl4 was measured at elevated temperature. Although heating produced a broadening of peaks, even at a temperature of 115 °C the meso resonances had failed to coalesce. This is the upper temperature limit experimentally possible so these experiments were not pursued further.
To explain the isolated product, 121, from reaction of excess bipyridine with 118, a solution which by NMR contained largely the 1:1 complex and bipyridine was passed through a short silica column, eluting with DCM/hexane (1/1). The eluent was found to contain exclusively 121, as the polar bipyridine is retained on the column thus shifting the equilibrium of equation 1 to the left. Although exchange is slow on the NMR chemical shift time-scale, on a ‘preparative chemistry’ time-scale the equilibrium is rapidly established. A similar effect was observed during recrystallizations. Recrystallization of solutions containing the 1:1 complex and excess bipyridine from chloroform layered with methanol removed the excess bipyridine which is soluble in the methanol. The porphyrin material which crystallized thus contained an enhanced proportion of 121.
The X-ray structure of 121 was obtained (figure 2.10) and the molecular connectivity is identical to the structure proposed for the compound in solution. The porphyrin units are slightly saddled252 and the bipyridine adopts a planar conformation. Although on steric grounds repulsion between the H3 protons should favour a non planar bipyridine conformation the barrier to coplanarity is slight. An electronic effect from coordination to the porphyrins might increase conjugation between the pyridyl rings thus favouring the planar conformation. Alternatively a preferred orientation of the ligand with respect to the porphyrin which minimizes steric clash, as has been proposed for imidazole,253,254 could also lead to the observed geometry.
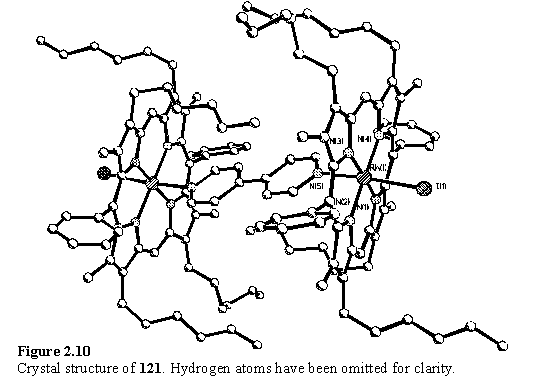
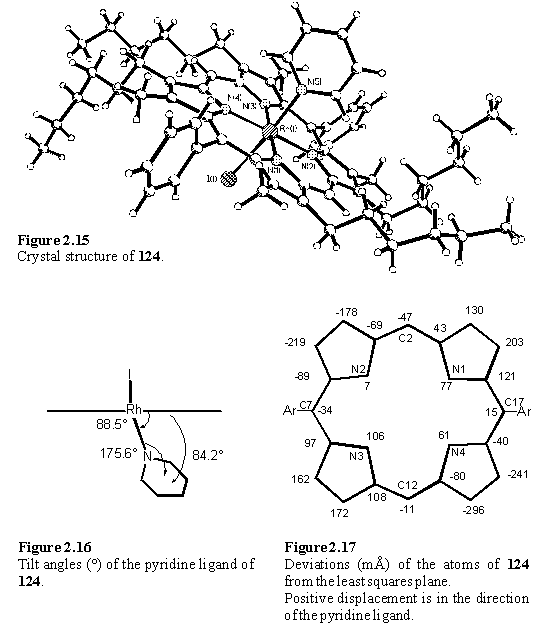
The bipyridine ligand is tilted from the perpendicular to the porphyrin plane and the angle between the best fit porphyrin and pyridyl planes is 71.0(1)°. The tilting arises from a combination of a tilt of the N5-Rh1 bond from the pyridine plane by 14° and of N5-Rh1 from the perpendicular to the porphyrin plane by 5°. The tilt angles and porphyrin deviations are illustrated in figures 2.11 and 2.12. Similar tilted structures have been reported for coordination oligomers of Ru130,255 and Zn132,158-160,165,166 pyridyl porphyrins and in these cases the tilt was ascribed to strain160 and crystal packing effects.130 Tilting serves to reduce the distance between the porphyrin planes which may assist in the formation of a compact structure lacking voids. Another example of a tilted structure is presented in section 2.4.6.
Attempts were made to dissociate 121 into the monomeric porphyrin constituents, as a model for a templated and covalently linked porphyrin dimer.246 Addition of TFA to a solution of 121 in chloroform produced a colour change from orange to deep green. It was hoped that washing with water would remove protonated ligand. On washing the orange colour was recovered but the NMR spectrum demonstrated that the starting material was almost unchanged. The identity of the species responsible for the green colour is unclear. The porphyrin was not demetallated so a porphyrin dication in which the pyrrolic nitrogens are protonated cannot be the origin of the colour. The harsher conditions of TFA in refluxing chloroform for 5 minutes converted 121 to an intractable mixture containing no starting material or 118. It would appear that the porphyrin is protonated by TFA, but at a site other than the pyrrolic nitrogen, and that this protonated species then undergoes decomposition reactions.
Rh(III) alkyl porphyrins are known to have lower affinity for nitrogen donor ligands then their Rh(III)-halo counterparts.222,223 Reaction of 121 with MeLi (scheme 2.4), followed by chromatography permitted isolation of monomeric alkylated porphyrin 123. The NMR of this product resembles that of ammonia complex 119, with the RhCH3 resonance appearing at -6.06 ppm. However in this instance the peak is a doublet with J = 3 Hz, due to coupling to the 100 % abundant spin -½ nucleus 103Rh.
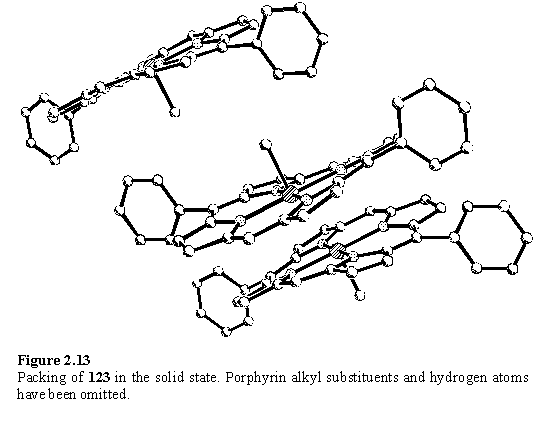
X-ray analysis of crystals grown from a methanol containing solvent mixture shows a solid state structure (figure 2.13) with 5 coordinate Rh. As in the solvent free structure of 118 this permits a close approach of the porphyrin planes to a distance of approximately 3.5 Å. The Rh-C bond length is 2.010(5) Å compared to a value of 2.031 Å reported for (OEP)Rh(III)Me.256
2.4.2 Rh(III) porphyrin pyridine complex
For comparison with 121 a simple Rh porphyrin pyridine complex, 124, was prepared by reaction of 118 with pyridine and evaporation of excess pyridine in vacuo. Chemical shift and Dd values for the pyridyl protons are given in figure 2.14. The Dd values for the a and b protons are almost identical to those for the 1:1 complex of 118 with bipyridine (figure 2.8) indicating a similar geometry of the ligand with respect to the porphyrin for both these species in solution.
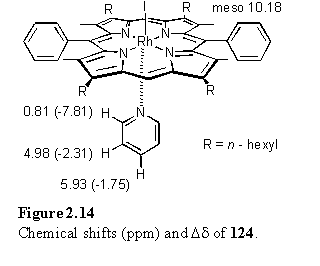
A titration of 118·MeOH at a concentration of 5.5 mM in DCM with pyridine was monitored by UV/visible spectroscopy with the aim of determination of the association constant of the complex. Processing of the spectra over the range 300 - 600 nm using the program Specfit,257 which performs a global fit of the spectra to a user defined model, returned a value of 108 M-1 for the association constant. This can only be regarded as an estimate of the binding constant due to the extreme sharpness of the titration curve combined with the experimental uncertainties.258
Contrasting to the solid state structure of 121, in that of 124 (figure 2.15) the pyridine plane is almost perpendicular to the porphyrin, with an angle of 84.2(1)° between the best fit porphyrin and pyridine planes. The tilt angles are illustrated in figure 2.16. A perpendicular ligand orientation is likely to be the most energetically favourable in an isolated molecule and in this case efficient crystal packing is achieved whilst maintaining this geometry. The Rh-Nax bond lengths in 121 and 124 are 2.121(4) Å and 2.139(3) Å respectively. The porphyrin distortion of 124 (figure 2.17) is of the same type (saddle) and magnitude to that of 121, although it is difficult to conclude that this is due to the effect of ligand coordination and not packing forces.
2.4.3 Rh(III) porphyrin 4,4'-bipyrimidine complex
It was decided to investigate ligands with both chelating and bridging coordination modes as these could potentially bridge between porphyrins, whilst simultaneously coordinating a third metal at the chelating site. Porphyrin coordination at the chelating site is prevented on steric grounds. The ligand 4,4'-bipyrimidine, 125, was attractive in this respect as it presents divergent nitrogen atoms resembling those of 4,4'-bipyridine and chelating nitrogens offering the diversity of coordination chemistry available to 2,2'-bipyridine.
This ligand was not commercially available and a variety of routes have been described for its synthesis. These can be classified into a multistep route starting from acyclic precursors,259 and methods involving coupling of pyrimidine or its derivatives.260-262 Of the latter category, a route via coupling of lithiated pyrimidine262 (scheme 2.5) appeared preferential to pyrolytic261 or electrochemical methods.260 125 was prepared satisfactorily using this method and was reacted with 118 to form dimeric porphyrin complex 126 using the same conditions used to prepare 121. Presumably this also proceeded through the initial formation of a 1:1 complex which was subsequently converted to the observed product on column chromatography.
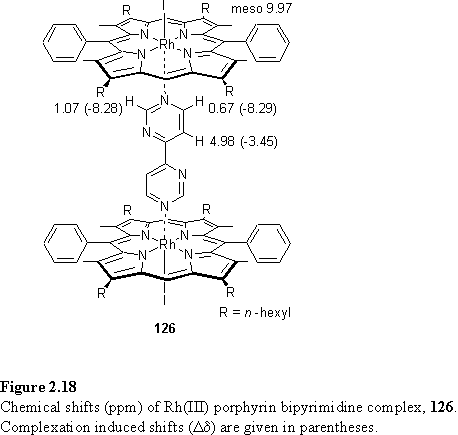
As expected from the similarity of the geometries of 4,4'-bipyridine and 125 the 1H NMR resonances of the ligands of 121 and 126 (figure 2.18) experience similar shift differences (Dd) from those of the free ligand.
The X-ray structure of 126 (figure 2.19) obtained from crystals grown under the same solvent conditions used to crystallize 121, is almost identical to that of 121. Both the unit cell and arrangement of its contents, including a solvent molecule, are common to each structure. The ligand is ‘buried’ between the porphyrins and appears to have made no difference to the conformation and packing of the molecules.
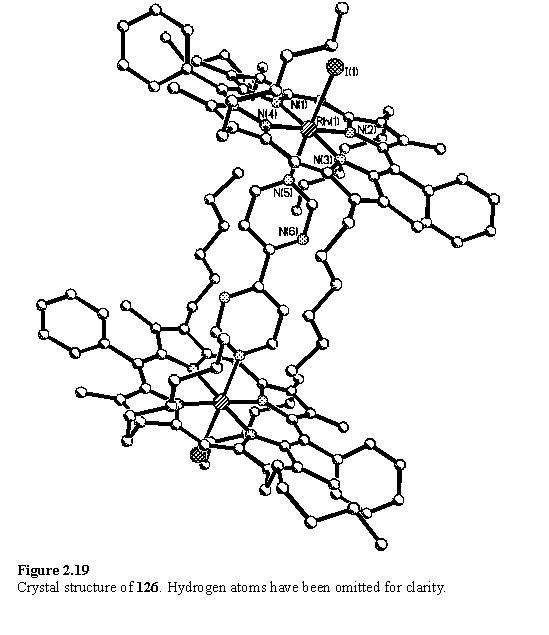
Literature reports indicated the ability of 125 and a methyl substituted derivative to simultaneously coordinate metals, such as W(0)263 and Cu(I)264 in bridging and chelating modes. Thermal substitution of CO was used to prepare the previously described Mo(0)265 and Re(I)266 complexes, 127 and 128. Initially, by tlc no reaction was observed between either of these complexes and 118, contrasting with the formation of 121 and 126 which appeared as new spots on the tlc plate. 1H NMR spectra of mixtures of 127 or 128 with 118 were complex showing several meso peaks, porphyrin bound and free bipyrimidine, and could not be rationalized in terms of the envisaged 1:1 or 1:2 complexes. Some decomposition of both porphyrin and the bipyrimidine species appeared likely although the identity of the products and mechanism of formation is not known.
Re(CO)5Cl was refluxed in THF overnight to thermally replace CO with labile THF ligands,267 then reaction with 126 at 35 °C for 11 h afforded a monomeric porphyrin product, as judged from a meso resonance at 10.28 ppm. There appeared to be no bipyrimidine bound, suggesting this type of reaction may have potential for removal of bipyrimidine after templated synthesis of rhodium porphyrin oligomers.
These results indicate that chelation of 125 by Mo(CO)4 and Re(CO)3Cl fragments reduces its ability to act as a bridging ligand to Rh(III) porphyrin. From the examination of the X-ray structure of 126 it appears unlikely that chelation would sterically interfere with porphyrin coordination, so an electronic effect which reduces the availability of the 4,4' nitrogen atoms for coordination to Rh would seem to be the reason for the failure of these reactions.
2.4.4 Rh(III) porphyrin diazapyrene complex
After the observation of the similarities between the solution and solid state structures of 121 and 126 it was decided to investigate the reaction of diazapyrene, 131, with 118. Diazapyrene was expected to bridge between two porphyrins giving a metal - metal distance comparable to 121 and 126, but unlike bipyridine and bipyrimidine, is constrained to planarity and capable of filling a slightly greater proportion of the space between the porphyrins.
The preparation of diazapyrene originally described in the literature268 is displayed in scheme 2.6. The final step involving molten Se at a temperature exceeding 300 °C is hazardous and so a more recent synthesis269 in which this step was replaced by a treatment with Pd/C appeared superior. This latter synthesis also claimed preparation of bis(N-methylimide), 129, from 1,4,5,8-naphthalenetetracarboxylic anhydride as an alternative to the tetracarboxylic acid in scheme 2.6. At attempt at preparation of 131 using the procedure described in reference 269 failed to yield any of the desired product, an observation which is shared with others.270 However, 129 was successfully obtained according to reference 268, and reduced to 130 with LiAlH4.271 This material was heated with Pd/C and from the residues 131 was isolated in poor yield and purity.
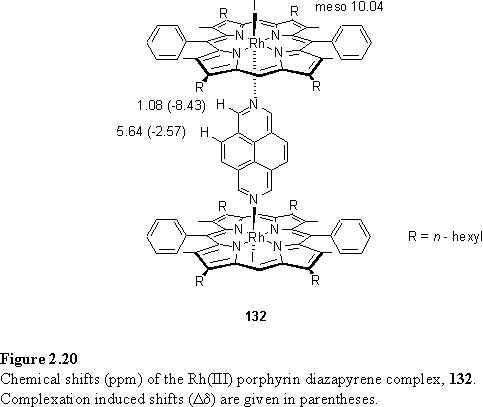
Reaction of 118 with 0.5 eq 131 followed by chromatographic purification afforded bridging complex 132 as a bright orange powder. The 1H NMR spectrum, and complexation induced shifts (Dd) are given in figure 2.20. Dd for Ha is indeed close to its value in 121 and 126 attesting to its similar location with respect to the porphyrins in the conformations accessible in solution.
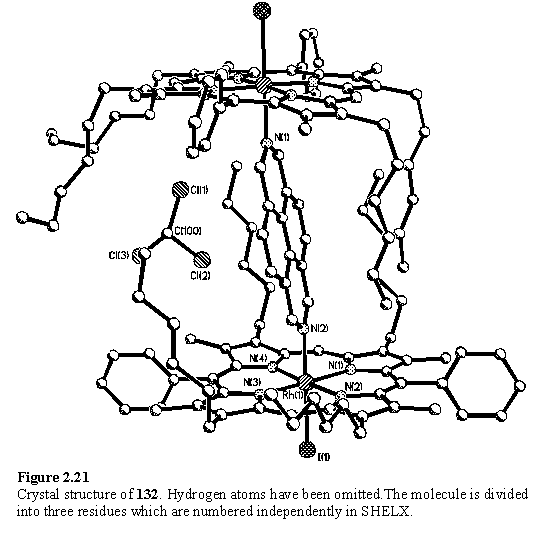
The X-ray structure of 132 (figure 2.21) is very different from that of 121 and 126 in that the two porphyrin units are not aligned with their aryl substituents parallel, but are rotated 90° with respect to each other. The molecules are packed in such a way that the hexyl chains are folded around the diazapyrene group and a chloroform solvent molecule. The plane of the diazapyrene group is almost perpendicular to the porphyrin plane and the a protons are oriented between the pyrrole nitrogens in the conformation proposed to give rise to minimum steric clash.253 The Rh-Nax bond lengths of 2.115(4) and 2.091(4) Å are almost identical to the values in 121 and 126 and the NMR data indicate that the complexes are also geometrically similar to each other in solution.
2.4.5 Nitrile coordination to Rh(III) porphyrin
The coordination of nitriles to Rh porphyrin was investigated to determine the potential of this functional group to assemble the porphyrins into designed structures. To date the literature reports an example of an organometallic Rh porphyrin nitrile coordination polymer, although coordination of the nitrile in solution was claimed to be weak.272
For the current work acetonitrile was chosen as the case for initial study. Titration of 118·MeOH in DCM at 4 mM with MeCN caused the disappearance of the Soret band at 402 nm and appearance of a new band at 420 nm, as MeCN coordinated to the porphyrin. However addition of several thousand equivalents of MeCN was required before no further change in the UV spectrum was observed, indicating comparatively weak binding relative to that observed for pyridine.
The titration was repeated at an initial porphyrin concentration of 6 mM in CDCl3 and was monitored by 1H NMR spectroscopy. As the titration progressed the MeOH resonances were observed to shift downfield, but after addition of 5 eq of MeCN still had not reached the values expected for MeOH in CDCl3. Evidently the MeOH effectively competes with MeCN for coordination to Rh. The MeCN resonance appeared initially at < 1 ppm and shifted downfield, implying fast exchange of free and bound MeCN on the chemical shift time-scale, contrasting sharply with pyridyl ligands. The spectra were further complicated by downfield shifts of another singlet peak, which was assigned to water also in fast exchange between free and coordinated forms. The spectra and peak assignments are displayed in figure 2.22. Unfortunately the presence of multiple competing ligands prevents quantitative analysis of these data without further information. However an important conclusion that can be drawn is that if axial ligation of nitriles is to be a successful means of organizing Rh porphyrins such as 118 then coordinating solvents such as alcohols must be avoided.
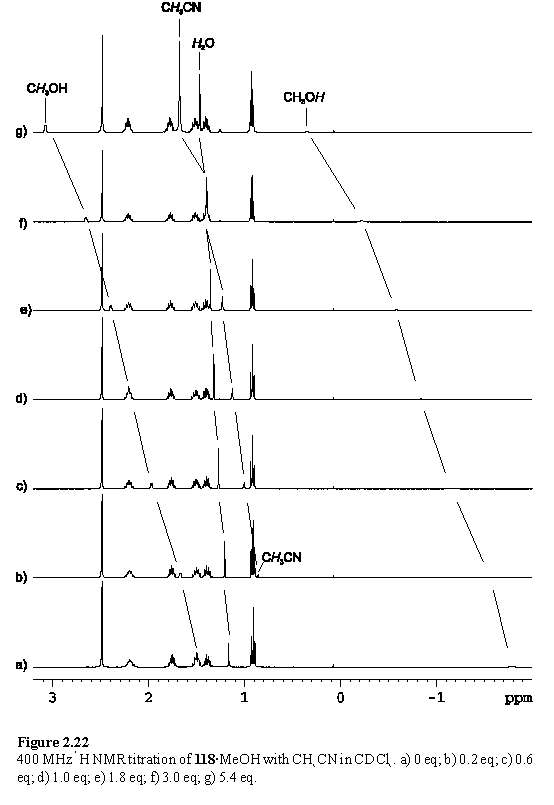
Crystals of 118 containing coordinated acetonitrile were grown from a toluene solution of 118 layered with MeCN and the structure is shown in figure 2.23. There are two porphyrin molecules in the asymmetric unit, although they both share the same structural characteristics. Saddle distortions are again observed and the axis of the coordinated acetonitrile is almost perpendicular to the porphyrin plane. The Rh-Nax bond lengths of 2.075(14) Å and 2.062(14) Å are slightly shorter than the corresponding lengths in the structures of 119, 121, 124, 126 and 132 although the significance of this is marginal given the large estimated standard deviations on the former measurements.
2.4.6 Rh porphyrin complex with 5,5'-dicyano-2,2'-bipyridine
As the experiments with acetonitrile demonstrated that the nitrile group does bind to 118, albeit weakly, it was decided to follow up this work by investigating multidentate nitrile ligands.
The 5,5'-dicyano-2,2'-bipyridine ligand, 137, was chosen as it offers diverging nitrile groups accessible to porphyrins, and chelating nitrogens providing the scope for further functionalization with metal complexes. Simultaneous bridging and chelating modes of coordination to Ag(I) in the solid state have been described in the literature.273
137 was prepared using a series of literature methods (scheme 2.7). 3-Picoline was coupled using freshly prepared and dried Raney Ni274,275 to afford 5,5'-dimethyl-2,2'-bipyridine, 133, although this compound has subsequently become commercially available. Jones oxidation,276 esterification277 and ammoniolysis273 afforded carboxamide 136.
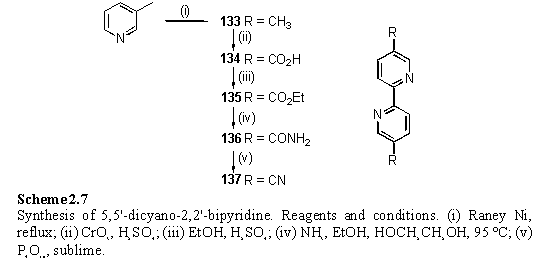
For preparation of the small quantities of 137 required for experiments the original literature procedure278 involving sublimation of 136 from P4O10 was selected in preference to alternative routes for effecting this conversion which involved sonication in POCl3,279 or dehydration with trifluoroacetic anhydride.273,280 Sufficient 137 was obtained from this approach, although in poor yield and not analytically pure.
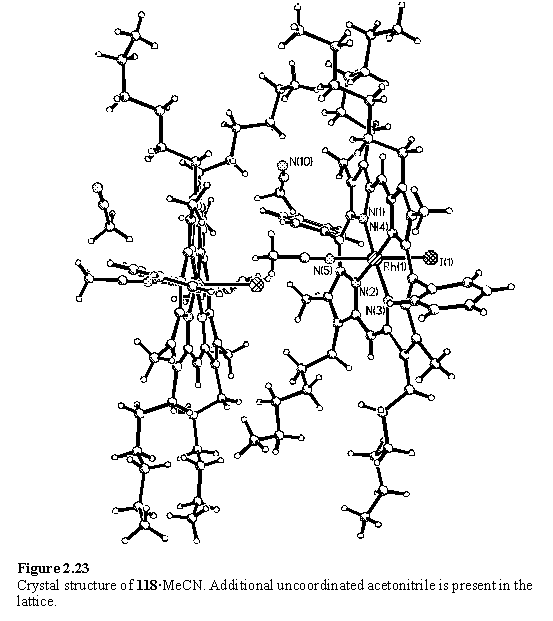
In the light of the NMR results for coordination of acetonitrile to 118 in solution, it was decided to cocrystallize 118 with 137 from non-coordinating solvent mixtures. 118 was filtered through a plug of silica gel, eluting with DCM, to try to remove MeOH, before addition of 0.4 eq of 137. Crystals were obtained slowly from a DCM/cyclohexane solution and the molecular structure of the complex, 138, is shown in figure 2.24.
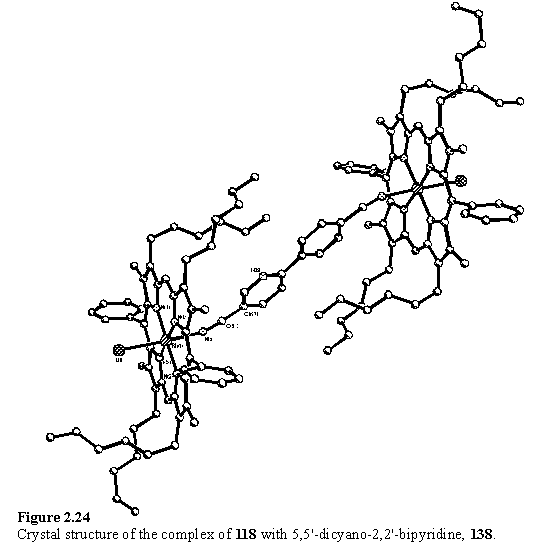
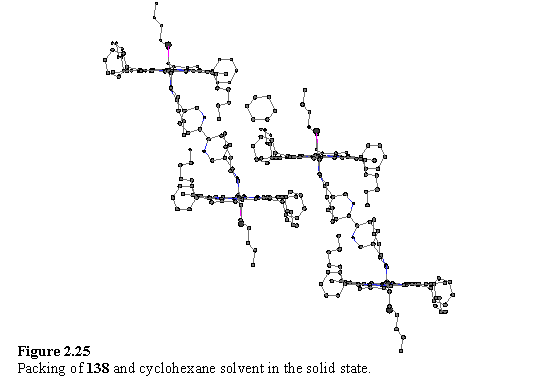
The axis of the bipyridyl group is severely tilted with respect to the normal of the porphyrins, which gives rise to a reduced separation between the porphyrin groups and a lateral offset. The ‘pivot point’ is predominantly the nitrile N atom and the bond angle Rh(1)-N(5)-C(61) is 155.9(2)°, although the nitrile group itself is slightly bent with an N(5)-C(61)-C(62) angle of 171.8(3)°. It is proposed that the potential energy function for bending Rh-N-C from 180° has a gentle slope permitting tilting of the ligand from an in vacuo energy minimum at perpendicularity to allow for optimal packing in the solid state. A packing plot (figure 2.25) shows that the molecules of 138 are interdigitated with each other, and that a cyclohexane molecule occupies the remaining space between the porphyrin units.
The 5,5'-dicyano-2,2'-bipyridine ligand lies on an inversion centre so the N-C-C-N torsion angle between the pyridyl groups is exactly 180° by symmetry. This conformation permits coplanarity, and hence increased conjugation, whilst avoiding a steric clash between the 3 and 3' hydrogen atoms.
The porphyrin is distorted into an irregular saddle shape, and the magnitude of the atomic deviations from the best fit plane are smaller than those of the acetonitrile complex. Jentzen et al.281 have analysed distortions of porphyrins in crystal structures using a normal mode model and found that the commonly observed distortions can be described as the sum of static displacements along the lowest frequency normal modes of the macrocycle. They calculated that the saddle type mode possesses the lowest frequency and it is this distortion which has been encountered most frequently in the Rh porphyrin structures presented here. The factors responsible for the sense or magnitude of the distortion appear to be subtle and in the current examples it does not appear possible to relate them to any steric or electronic features of the ligands or crystal packing.
As with 118·MeCN, the Rh-Nax bond length of 2.054(2) Å is shorter than in the pyridyl complexes, perhaps contradicting the expectation that this bond would be weaker and longer, on the basis of the relative ligand affinities and labilities in solution. This difference could arise from the nature of the donor orbital, the nitrile lone pair having a greater proportion of s character than that of pyridine, leading to a shorter bond length but poorer donor ability of the former. A steric effect could also be involved since the nitrile lacks the Ha protons which make a close approach (~2.4 Å in 124) to the porphyrin plane.
2.4.7 Rh porphyrin DABCO coordination
In general, aliphatic amines have been shown to bind more tightly than pyridine to Zn porphyrins.282 Both Zn283 and Ru284 porphyrins have been shown to form ‘sandwich’ complexes with the bidentate amine ligand diazabicyclooctane (DABCO) and this interaction has been used to fold dendrimers containing Zn porphyrin units.285
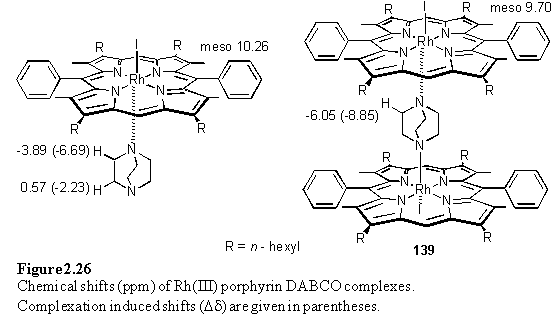
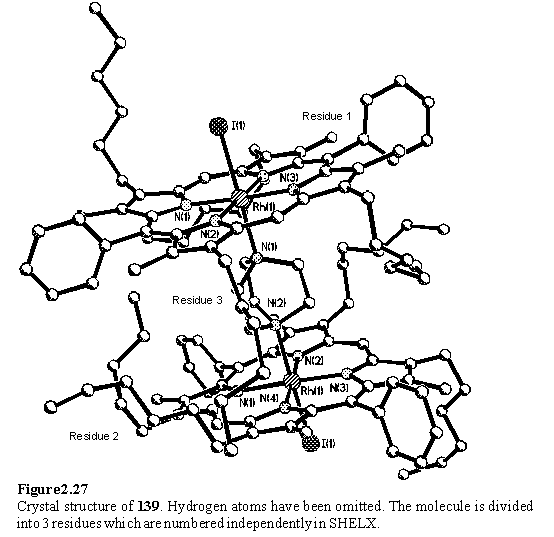
NMR titrations of 118·MeOH in CDCl3 with DABCO enabled the chemical shifts of the dimeric complex, 139, and the 1:1 complex to be determined (figure 2.26). All species were in slow exchange on the chemical shift time-scale at room temperature. The Dd values are not quite additive, possibly due to binding of a second porphyrin altering the disposition of the DABCO protons relative to their positions in the 1:1 complex. The cooperativity coefficient a has been calculated from the integrals of the resonances of DABCO, both free and in the two complexes. From two independent determinations values of a = 92 and a = 78 were obtained implying that binding of one porphyrin disfavours binding of the second. These values are larger than those reported by Anderson for DABCO binding to Ru(II)CO mesoporphyrin(II)-dimethyl ester.284 It is clear from the X-ray structure of 139 (figure 2.27) that the substituents of the porphyrins can come into contact with each other. This restricts the conformations available to the substituents and may entropically disfavour binding of the second porphyrin. The Rh-Nax bond lengths of 2.266(9) and 2.254(10) Å are longer than those observed for Rh-NH3 and Rh-Pyridine.
As was observed for 4,4'-bipyridine, passage of the 1:1 complex through silica gel, eluted with DCM/hexane (1/1) retained excess ligand on the silica, thus displacing the equilibrium towards the dimeric complex.