2.3 Rh(III) meso-oxo porphyrin
During the course of the work described in section 2.2, a bright yellow band was observed to rapidly elute from columns during purification of 118. This trace product, 120, was collected, but a firm identification could not be made from the 1H NMR spectrum. This lacked the typical porphyrin meso resonance at around 10 ppm, but did indicate the presence of phenyl groups and hexyl chains suggesting an intact porphyrin-like skeleton. A low resolution MALDI mass spectrum gave peaks at m/z 1230 and 972.5 assigned to [M]+ and [M-2I]+. Careful crystallization yielded a sample suitable for X-ray analysis which revealed the structure of 120 to be an unusual oxidized porphyrin derivative with a carbonyl group at a single meso position and two iodo ligands coordinated to rhodium (figure 2.6).
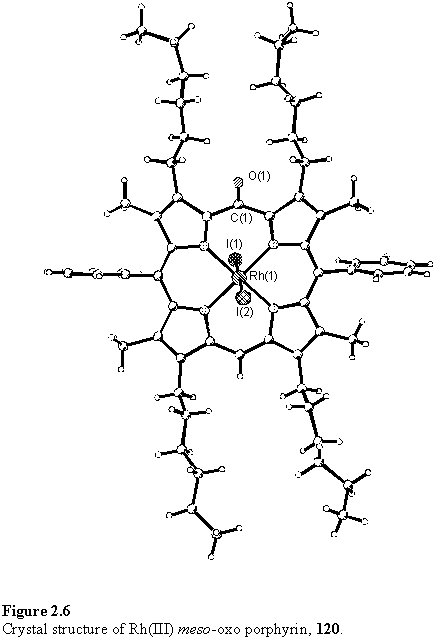
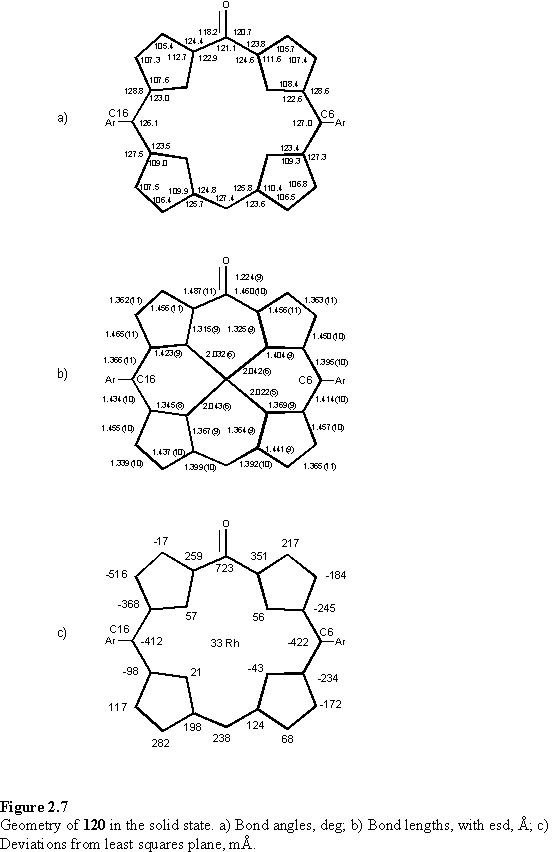
The diamagnetic nature of the compound requires a rhodium oxidation state of +3, and the C-O bond length of 1.224(9) Å is consistent with a double bond indicating the oxidation is entirely localized at this site. To accommodate the carbonyl group the bond angles at this meso carbon are distorted to around 120° contrasting to a C-Cmeso-C angle of 128.4(6)° in the crystal structure of the methanol solvate of 118 (figure 2.3). The carbonyl group interrupts the aromaticity of 120, which is manifested as a lengthening of the C-Cmeso bond lengths relative to 118 and the appearance of the remaining meso proton resonance at 5.02 ppm, characteristic of alkenes. 120 is significantly distorted from planarity, most likely as a consequence of these geometric changes. Relevant bond angles, lengths and deviations of the porphyrin from planarity are given in figure 2.7.
A search of the literature brought to light a similar Fe(III) compound which was synthesized by reaction of two molar equivalents of bromine with Fe(III) octaethyloxaphlorin dimer.243 A carbonyl stretching band in the IR spectrum of this compound was observed at 1734 cm-1, although a spectrum of solid 120 did not show this feature. A band was observed at 1631 cm-1 but this is outside the normal range of even diaryl and ab,a'b' unsaturated ketones.244
The mechanism of formation of 120 remains unclear. The action of iodine as an oxidant on 118 was considered as a possibility, although reaction of iodine for 17 h with 118 in DCM under air in the presence or absence of NaOAc followed by an aqueous work-up failed to afford any 120. The unidentified product of this reaction appeared to be paramagnetic, displaying broadened proton resonances up to 23.4 ppm.