1.3 Order in two dimensions
Surface coordination chemistry may be used to generate chemically well defined, functionalized and spatially patterned surfaces. There is the opportunity to create molecular order both perpendicular to and in the plane of the surface, on length scales from the atomic to the macroscopic.
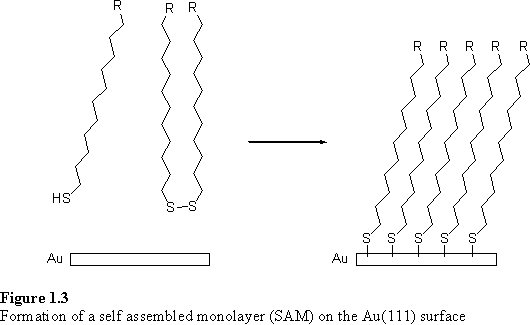
A popular system which has been extensively exploited for the preparation of functionalized surfaces is the coordination of sulfur compounds, notably thiols and disulfides, to gold to form self assembled monolayers (SAMs).17 The adsorption takes place spontaneously when a clean gold surface is exposed to thiol/disulfide in the gas or solution phase. Many alkyl thiols and disulfides pack in a two dimensional crystalline lattice on the Au(111) surface.18 Alkyl thiols with terminal functional groups such as carboxylic acids and alcohols bind to the gold selectively through the thiol moiety, leaving the functionality exposed at the upper surface of the monolayer (figure 1.3).19
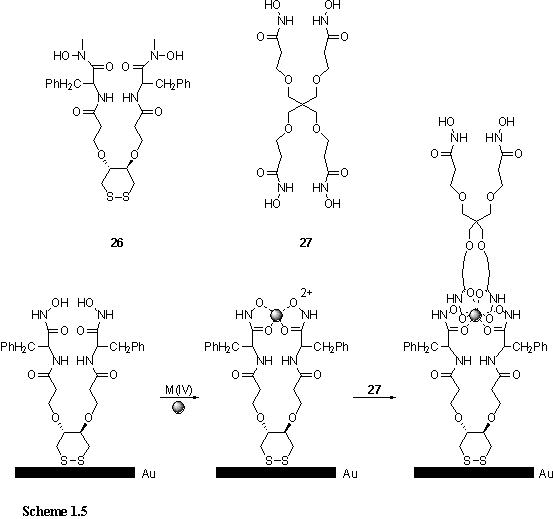
The work of Shanzer et al. provides an example of the creation of an ordered multilayer structure using coordination chemistry (scheme 1.5).20 The bis-hydroxamate ligand, 26, was anchored to a gold surface through a disulfide moiety to form a self assembled monolayer. The hydroxamate groups chelate to the large tetravalent and eight coordinate ions Ce(IV) or Zr(IV), and this step was carried out by dipping the monolayer functionalized gold substrate into solutions containing these metals. Dipping into a solution of ligand 27 completed the coordination shell of the metal, and provided another bis-hydroxamate site at which a further metal could be coordinated by another dipping cycle. In this way a multilayer film with Zr(IV) or Ce(IV) at well defined depths was prepared. Evidence for the film growth was provided by the increasing ellipsometric thickness, and an oscillation in the water contact angle between successive metal and ligand dipping cycles. Films containing both Zr and Ce were analysed by XPS, the results of which were consistent with the elemental depth profile that would be predicted from the dipping sequence.
In addition to the in-plane order created by two dimensional crystallization in self assembled monolayers, it is possible to chemically pattern monolayers with thiols differing in their terminal functional group. A number of methods have been described recently for fabrication of chemically patterned surfaces with minimal use of photolithographic procedures which require specialized and costly equipment.
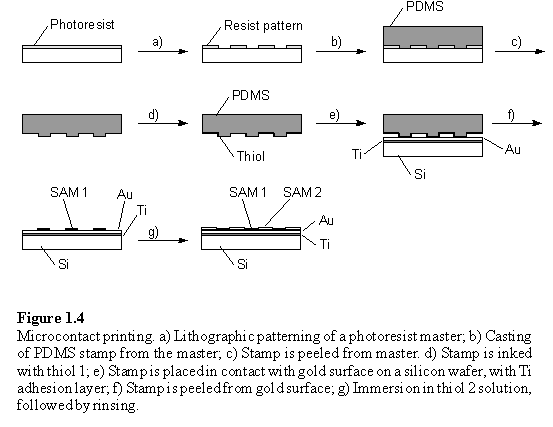
Whitesides et al. have developed a technique which they call microcontact printing (mCP) for the preparation of patterned monolayers (figure 1.4).21,22,23 A polydimethylsiloxane (PDMS) stamp with a surface relief pattern is ‘inked’ with thiol and placed on a gold substrate. The thiol is transferred to the surface where the raised parts of the stamp make contact, and forms a monolayer on these areas. The stamp is peeled from the surface, which may then be immersed into a solution of a second thiol to form a different monolayer on the remaining regions of bare gold. The stamps themselves are cast from a master, which requires lithographic fabrication, but once a master is available no further access to lithographic facilities is required. The stamps may be used many times for printing of the same compound, and can print sub-micrometre sized features.24 The same technique has been used to pattern other types of monolayer, for example alkanephosphonic acids on the native oxide layer of aluminium films.25
An alternative approach to micro and nano fabrication of monolayers is to use a scanning probe microscope tip to locally modify the surface. This has the disadvantage that it is a serial technique; the tip must write the features of the pattern individually, whereas mCP deposits all of the pattern simultaneously. However the writing approach has the flexibility that the pattern may be changed at will using the microscope control software.
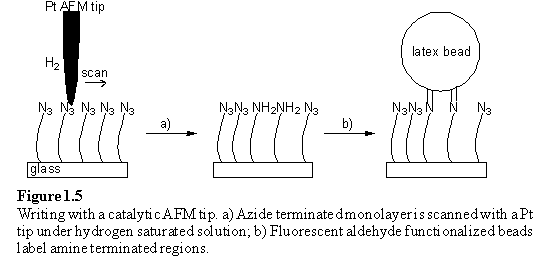
Schultz et al. prepared an alkyl azide monolayer on a silicon surface and scanned this surface with a Pt coated AFM tip under hydrogen saturated isopropanol (figure 1.5).26 The tip acted as a local hydrogenation catalyst which reduced the azide to amine in the scanned area. To enable visualization of reaction, the surface was treated with either aldehyde functionalized fluorescent latex beads, or 3-(2-furoyl)quinoline-2-carboxaldehyde. The latter compound reacts with primary amines to form a fluorescent isoindole. Fluorescence microscopy demonstrated that only the areas scanned with the catalytically active Pt AFM tip were fluorescence labelled, scanning with a Si3N4 tip did not result in reduction of the azide groups.
Instead of using the AFM tip to perform a surface reaction, other workers have used a tip to deliver molecules to a surface. When imaging in air a meniscus of condensed water exists at the point of contact of the AFM tip and a surface,27 and it has been found that it is possible to transport molecules from the tip to the surface via this meniscus.28 A self assembled monolayer could be written by scanning a gold surface with an AFM tip coated with octadecanethiol (figure 1.6) and the patterned surface could be imaged with the same tip by scanning at a high speed so that negligible deposition of thiol occurred. The name ‘dip-pen nanolithography’ (DPN) was coined for this method of writing on surfaces. The role of the water meniscus in the transport of the thiol was evident from the increase in the linewidth and speed of monolayer formation as the humidity was increased. It was possible to pattern several thiols on the same surface by scanning with a series of AFM tips, each carrying a different compound, analogous to a multipen plotter.29

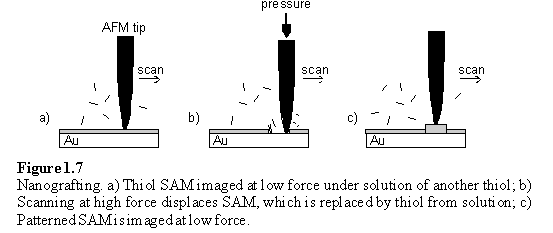
Mechanical displacement of thiol molecules from a monolayer using an AFM tip was carried out under a solution of a second thiol, which bound to the freshly exposed gold surface (figure 1.7).30 As with DPN, imaging of the resulting patterns was possible in situ by scanning with the same tip used for the manipulation but at a force too low to cause displacement of the monolayer.
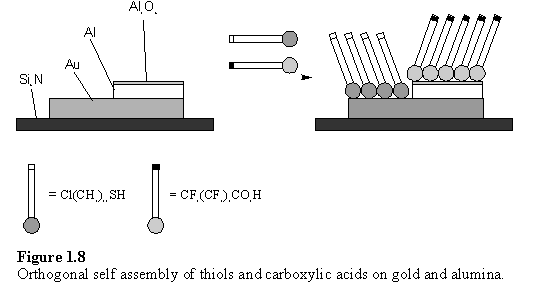
Selective coordination chemistry, using the hard/soft principle, has been applied to assemble monolayers of different compounds simultaneously onto surfaces patterned with different materials. This concept was termed ‘orthogonal self assembly’ (OSA) and was first demonstrated on a surface composed of wires of aluminium and gold on a Si3N4 substrate (figure 1.8).31 The aluminium was exposed to air so developed an oxide layer on its surface. This substrate was immersed into a solution 1 mM in both CF3(CF2)8CO2H and Cl(CH2)11SH for 24 h, followed by rinsing to remove physisorbed compounds. Auger microscopy element maps of F and S revealed that the ‘soft’ thiol had selectively bound to the Au, the ‘hard’ carboxylic acid was bound to the alumina regions and the Si3N4 regions were free of both compounds.
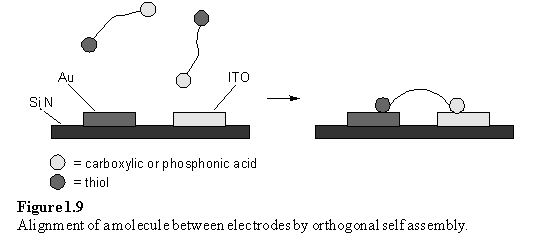
Indium tin oxide (ITO) is a more interesting substrate than alumina, as it is electrically conductive. Hence a combination of conductive ITO, Au and insulating Si3N4 was investigated as a substrate for OSA.32 The authors proposed that OSA could ultimately provide a means of ‘wiring up’ an oriented molecule between a pair of closely separated electrodes (figure 1.9). OSA combinations of thiol 28 and carboxylic acid 29, or thiol 30 and phosphonic acid 31 on a Si3N4 substrate supporting Au and ITO electrodes was shown to be successful by imaging mass spectrometry and Auger microscopy.
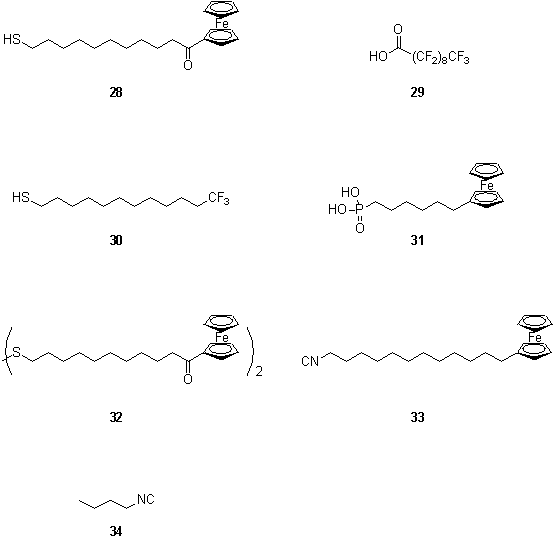
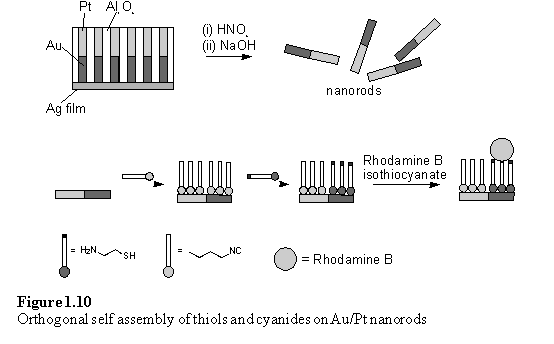
Wrighton et al. investigated the selectivity of organic disulfide 32 and isocyanide 33 for binding to Au and Pt surfaces.33 The composition of the resulting monolayers could be probed by cyclic voltammetry as the E½ values of the alkyl and acyl ferrocenes are sufficiently different that discrete redox waves are observed for each. It was found that the selectivity of the Pt surface for binding of isocyanide, 33, could be enhanced by pretreatment with an oxygen plasma. This treatment did not affect the selectivity of the Au surface, which bound 32 and 33 in a ratio of 8:1. This chemistry was applied by others to prepare colloidal metal ‘nanorods’ with selective surface functionalization (figure 1.10).34 Metal rods of 200 nm diameter and composed of segments of Au and Pt were electroplated into pores in an alumina membrane. After dissolution of the membrane to release the rods, these were treated with isocyanide 34 which formed a monolayer on both metals, followed by 2-mercaptoethylamine. The thiol displaced the isocyanide from the Au surface but left this monolayer intact on the Pt regions. The amino groups were tagged by reaction with the fluorophore Rhodamine B isothiocyanate. The rods were imaged by fluorescence and optical microscopy which showed the fluorophore to be localized on the gold portions confirming the success of the orthogonal self assembly procedure.