1.2 Self assembled systems with multiple building blocks
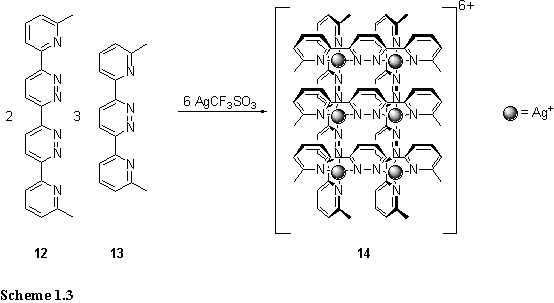
Structures of lower symmetry, and higher information content, require the assembly of several different ligands. Lehn extended the grid concept to the preparation of rectangular grids by the mixture of AgCF3SO3, 12 and 13 in a 6:2:3 ratio in nitromethane (scheme 1.3).12 The 1H NMR spectrum of the reaction mixture indicated 90 % yield of the 2 ´ 3 grid (14), 2 % of the 3 ´ 3 grid and 8 % of the 2 ´ 2 grid, deviating significantly from the statistical distribution of 48 %, 16 % and 36 % respectively. Selective formation of 14 was attributed in part to the poor stability of the 3 ´ 3 grid due to weak coordination in the central Ag(pyridazine)4 unit but also to solvation effects.
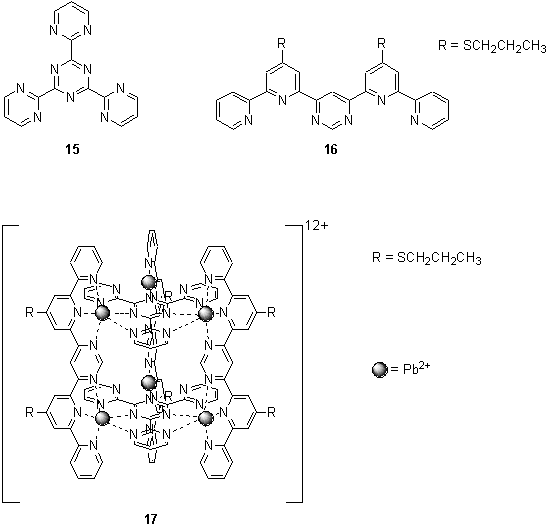
Likewise the cylindrical structure 17 was assembled from octahedral Pb(II) ions and tris-chelating ligands simply by stirring the triazine component, 15, with Pb(OTf)2 in acetonitrile for 2 h, followed by addition of 16.13 A 2 ´ 2 grid was observed as a minor side product in the formation of 17, especially if the incorrect stoichiometry of the components was used.
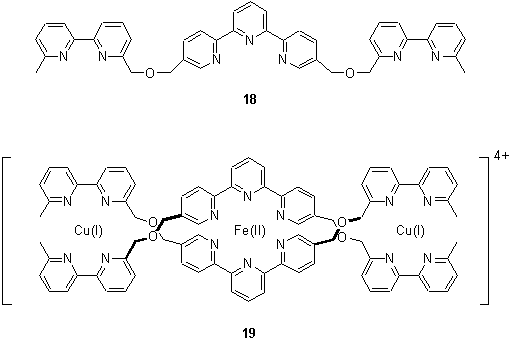
Combinations of metal ions with complementary properties such as different coordination geometry or ‘hardness’ may be used to direct the assembly of ligands in solution. The preference of Cu(I) for a tetrahedral environment and Fe(II) for an octahedral geometry permitted the assembly of the helicate 19 which was identified by ES MS.14 The order of addition of the metals to 18 was found to be unimportant, the same product being isolated regardless of whether Cu(I) or Fe(II) was added first. However if Cu(I) was added first heating was found to be necessary to obtain the product. This was believed to be due to the formation of species in which the Cu was coordinated to the tris chelating site and on heating these equilibrated to 19.
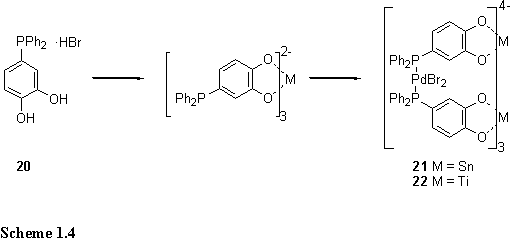
Raymond and Wong et al. exploited the hard/soft binding preferences of metals to construct an eleven component heterometallic structure.15 Catecholate is a ‘hard’ ligand which selectively binds high valent oxophilic metals such as Sn(IV) and Ti(IV), whereas phosphines show little affinity for these metals but bind to ‘soft’ Pd(II). Both donors were combined in a single ligand, 20, which was complexed first at the catechol site with Sn(IV) or Ti(IV), followed by reaction with PdBr2·2PhCN to yield the C3h symmetric products 21 and 22 in 95 % yield (scheme 1.4). The structure was confirmed by 1H, 31P, 13C NMR in solution and by single crystal X-ray crystallography in the solid state.
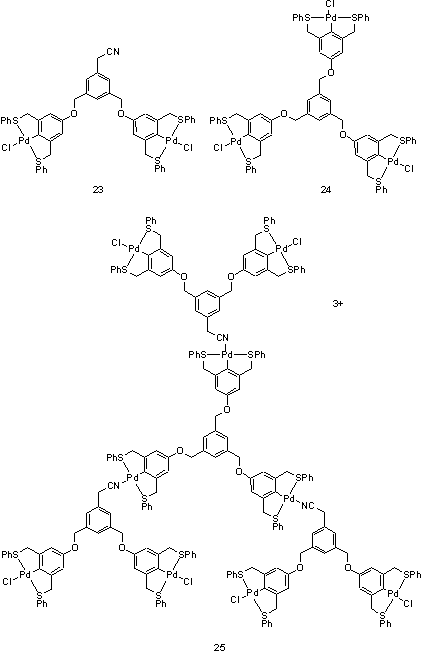
The structures discussed so far have all been closed, the final structure having no vacant coordination sites or free donor atoms so there is no scope for further enlargement. Huck et al. used a strongly coordinating chloride ion as an inorganic protecting group for Pd(II) pincer complexes on a dendritic wedge, 23.16 The chloride ion prevents the cyano group of the wedge from binding to the Pd centre. A threefold symmetric core, 24, was prepared and after replacement of the chloride ligands with non-coordinating tetrafluoroborate anions by reaction with 3 eq AgBF3, the wedge was added to yield a first generation dendrimer, 25. This could be activated by reaction with 6 eq AgBF4, followed by addition of 6 eq of 23 to afford the second generation dendrimer. Up to the third generation was prepared and characterized by NMR and ES MS.