Future work
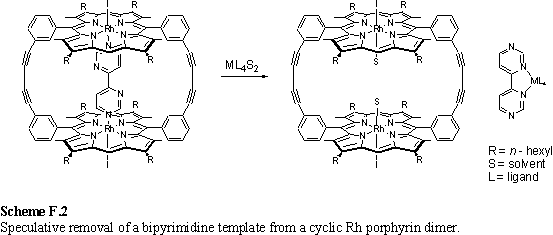
The Rh porphyrin coordination chemistry described in chapter 2 made use of only one of the axial coordination sites of the porphyrin. The iodo ligand was essentially inert and took no part in the chemistry. The scope of the structures available could be greatly increased by replacement of the iodo ligand with a group such as RS, RSe, RTe or R2N. This might be achieved by removal of the iodo ligand by reaction with 1 eq Ag+ followed by reaction with an appropriate anion to form a neutral complex according to scheme F.1. A further neutral ligand such as a substituted pyridine could then be coordinated.
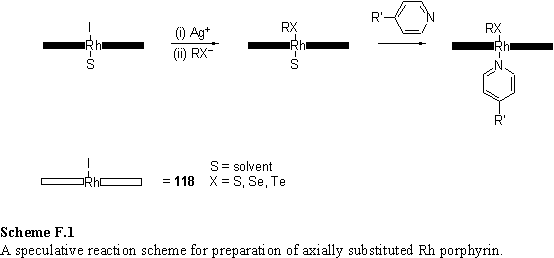
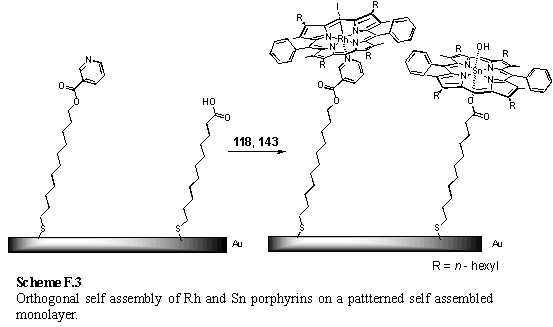
Another avenue of investigation would involve the use of Rh porphyrin coordination chemistry for templated synthesis of porphyrin oligomers. Previous work246 demonstrated the 4,4'-bipyridine templated synthesis of a Rh porphyrin dimer, but the ligand was too tightly bound to remove from the cavity of the dimer. In section 2.4.3 it was shown that 4,4'-bipyrimidine bound two Rh porphyrins, and thus should be capable of acting as a template for a covalently linked dimer. However it also appeared that binding of a metal at the chelating site of 4,4'-bipyrimidine disfavoured binding of porphyrins. Reaction of 4,4'-bipyrimidine, bound within a templated dimer, with a metal which coordinates to the chelating nitrogen atoms may release the 4,4'-bipyrimidine from the dimer (scheme F.2), thus permitting isolation of the empty dimer.
The orthogonal self assembly of Rh and Sn porphyrin onto carboxylic acid and pyridyl terminated microcontact printed SAMs (scheme F.3) still has to be attempted. A prerequisite is the preparation of contaminant and particle free patterned monolayers with terminal functional groups which are accessible for binding to porphyrins. Spatially resolved surface analytical techniques which yield compositional and structural information, such as imaging SIMS and XPS, are likely to be valuable tools for characterization of these systems. AFM also can be used to observe surface patterning with the advantage of the ability to image in situ modifications of the surface under a liquid environment.
Surface plasmon resonance is a technique which may be applied to measure the affinity of molecules in solution for receptors immobilized on a gold substrate. With suitable instrumentation (commercial instruments only operate with aqueous solvent) it may be possible to measure the affinity of metalloporphyrins in solution for ligand functionalized SAMs, or vice versa.
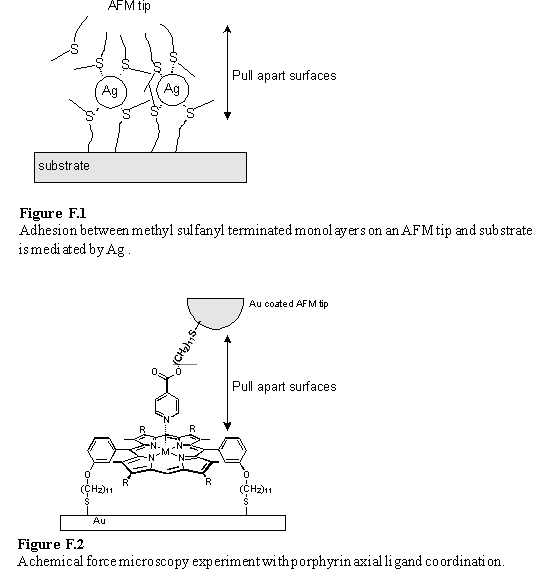
Alternatively AFM experiments may be used to measure binding between pairs of functionalized surfaces. In the chemical force microscopy472 experiment the adhesion force between a functionalized AFM tip and substrate is measured. The breaking of specific binding interactions between the contacting surfaces as they are pulled apart can contribute to the observed adhesion force. A relevant example taken from the literature is the Ag+ mediated increase in the adhesion between methyl sulfanyl terminated monolayers (figure F.1).473 This was ascribed to formation of a ‘sandwich’ type complex of silver ions between the two surfaces. If the concentration of Ag+ was increased further a fall in the adhesion force was observed and ascribed to electrostatic repulsion between surfaces both saturated with Ag+. It would be interesting to measure adhesion forces between ligand and porphyrin terminated SAMs (figure F.2) and try to correlate these with the solution phase binding affinities. Also the adhesion between pairs of porphyrin SAMs in contact with a solution of a bidentate ligand could be measured as a function of the ligand concentration. The behaviour of this system is expected to parallel that of the Ag+ / methyl sulfanyl system described in the literature.