4.4 Capacitance and heterogeneous electron transfer
An insulating monolayer on an electrode in contact with an inert electrolyte behaves as a parallel plate capacitor. Measurement of the charging current which is passed as the potential of the electrode is scanned enables calculation of the so called differential capacitance. As the monolayer thickness increases this is equivalent to increasing the separation between the plates of the capacitor and thus the differential capacitance decreases. The differential capacitances of monolayer modified electrodes at –0.35 V with respect to Hg/Hg2SO4 in 0.1 M NaClO4 electrolyte are presented in table 4.2.
|
Sample |
Differential Capacitance / mFcm-2 |
Cdl / mFcm-2 |
Rct / kW |
|
9.0 |
5.3 |
2.7 |
|
|
6.9 (0.9) |
4.2 (1.1) |
1.1 (0.7) |
|
|
rac 184 |
7.4 (1.2) |
4.2 (0.2) |
0.14 (0.04) |
|
(R) (-) 184 |
7.4 (0.9) |
4.6 (0.03) |
0.151 (0.009) |
|
5.1 |
CPE |
34 (8) |
|
|
4.9 |
CPE |
16 (6) |
|
|
5.5 (1.7) |
CPE |
22 (13) |
|
|
7.2 |
5.4 |
5.0 (1.3) |
|
|
8.8 |
5.7 (0.4) |
1.5 (0.2) |
|
|
6.4 (0.3) |
CPE |
3.6 (0.8) |
|
|
9.8 |
7.9 (0.7) |
0.8 (0.3) |
|
|
10.5 (1.5) |
7.6 (0.9) |
0.5 (0.1) |
|
|
12.7 (0.1) |
8.5 (0.4) |
0.304 (0.007) |
|
|
10.3 (1.1) |
7.3 (0.5) |
0.34 (0.05) |
|
|
Bare gold (THF) |
39 (12) |
20.3 (0.9) |
0.083 (0.015) |
|
Bare gold (EtOH) |
24.2 (1.7) |
23.0 (0.3) |
0.053 (0.007) |
Table 4.2
Differential capacitances from cyclic voltammetry (scan rate 0.1 Vs-1) and results from least squares fitting of impedance spectra of modified gold electrodes. Where more than one measurement was made standard deviations are given in parentheses. CPE indicates constant phase element behaviour. Samples of 180 and 181 used for these experiments were prepared using the method described in section 3.1.6.
Impedance spectroscopy measures the response (current and its phase) of an electrochemical system to an applied oscillating potential as a function of the frequency of this oscillation. The result of the experiment is the complex impedance, Z, as a function of frequency. Faradaic impedance spectra with a Fe(CN)63-/4- redox probe were modelled using the equivalent circuit approach of Randles438 using non-linear least squares fitting. In this approach the electrochemical cell is modelled as a circuit composed of resistors, capacitors, inductors and other components which have no analogues in conventional electrical circuits. The monolayer is treated as a resistor (Rct) and capacitor (Cdl) in parallel, and the solution resistance is represented by a resistor Rs. Representative complex plane impedance plots for bare gold and monolayer electrodes are shown in figure 4.5.
a)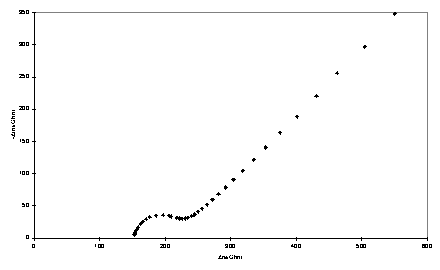
b)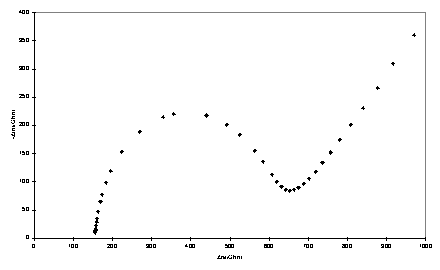
c)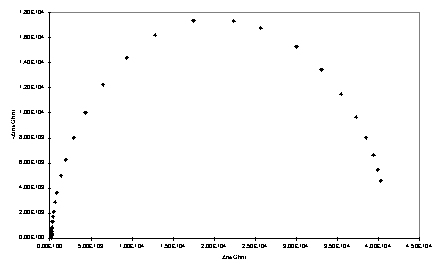
Figure 4.5
Complex impedance plane plots obtained with gold electrodes and adsorbed porphyrin thiol/disulfide. a) Bare gold; b) 234; c) 223.
Where possible the data were fitted to the circuit of figure 4.6 in which the parameters of interest are the capacitance, Cdl, and charge transfer resistance, Rct. The Warburg impedance, Zw, is a term arising from diffusion of the redox couple to and from the electrode. Its effect is noticeable at lower frequencies where it gives rise to a linear region with a slope of unity in the complex impedance plot. In some cases, 223 - 225 and 229, Cdl could not be modelled satisfactorily as a pure capacitance and a ‘constant phase element’ (CPE) was used instead. There is a precedent for constant phase element behaviour of monolayer electrodes439 and for blocked electrodes at potentials at which Faradaic processes are not operative this has been explained by the inhomogeneity of polycrystalline surfaces440 or fractal roughness.441
Deviations from ideal Warburg behaviour were also occasionally observed, manifested in the impedance plane plots as a slope of the linear region differing from unity. However the error in Zw will be large when over the frequency range of the measurements the contribution of diffusion to the impedance is relatively minor due to a large Rct.
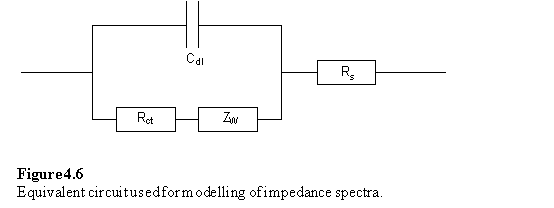
The results of the fitting procedure are also presented in table 4.2.
The differential capacitances of the monolayer electrodes are consistently lower than those measured at bare gold surfaces. A monolayer prevents approach of ions to the electrode surface and thus increases the capacitance. The values seem high when compared to monolayers of straight chain and terminally functionalized alkane thiols442,443 although these results were obtained with a different electrolyte. The effect of electrolyte on the capacitance of monolayer electrodes has been noted and ascribed to different degrees of penetration of the monolayer by hydrated ions.444 High capacitance values could indicate a thin monolayer or permeability to the electrolyte. The capacitance values derived from the impedance spectra mirror those obtained by CV, although the former are smaller in magnitude.
The magnitude of Rct varies greatly between monolayers of the different classes of compound. Charge transfer between SAM modified electrodes and a redox active species in solution has been proposed to occur at either pinhole defects at which the redox couple can directly contact the metal surface, or by tunnelling through ‘collapsed sites’.445 Regardless of the detailed mechanism of charge transfer, the samples with low Rct are less effective at preventing the redox probe from accessing the electrode. This could indicate a disordered layer, with only partial coverage of the gold surface, or a structure which is intrinsically porous.
Dithiol porphyrins 223 - 225 formed the most blocking monolayers, on the basis of highest Rct and lowest capacitance, contrasting to the disulfide strap compounds 234 and 235. This confirms the conclusions of the contact angle experiments that the structures of monolayers of these two types of compound are considerably different. The differences could arise due to binding of only a single thiol group of 223 - 225 to the surface whereas both arms of 234 and 235 could bind simultaneously. Slow kinetics of adsorption, desorption and monolayer rearrangement could prevent identical equilibrium structures from being attained. There is a literature precedent for monolayers containing free thiol groups, as observed by X-ray photoelectron spectroscopy, both for complex poly-thiols with porphyrin headgroups175 and for simple linear alkane dithiols.387 Alternatively there is the possibility of polymerization of the dithiol by oxidation, and adsorption of polymer to the gold surface leading to a disordered multilayer film. The preliminary XPS results described in section 4.1 appear consistent with this hypothesis. Further confirmation could be obtained by ellipsometric thickness measurements, or estimation of the quantity of adsorbed porphyrin from UV spectra.
Cyclic voltammograms of the monolayer electrodes with the Fe(CN)63-/4- redox couple displayed reduced peak currents and increased peak separations relative to a bare gold electrode. This effect is predicted from a model of partially blocked electrodes.446 Corroborating the results of the impedance analysis, the lowest currents were observed thorough monolayers of dithiol porphyrins, with monolayer electrodes of disulfide strapped porphyrins displaying a prominent Fe(CN)63-/4- redox wave. Representative CVs are given in figure 4.7.
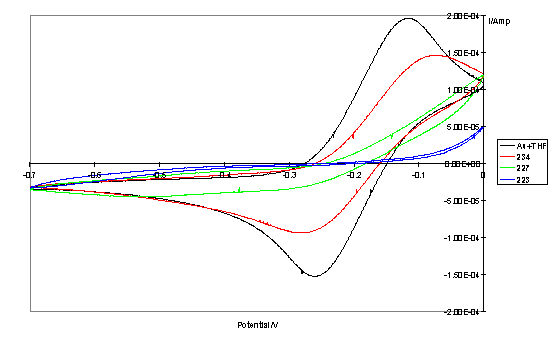
Figure 4.7
Cyclic voltammograms obtained with a bare gold control sample (treated with THF) and thiol/disulfide porphyrin functionalized electrodes and Fe(CN)63-/4- redox couple.
Repeated scanning of monolayers of 223 and 224 up to a potential of +0.8 V led to decreasing peak separations and increasing currents (figure 4.8) from which it can inferred that the monolayer is destroyed at these potentials. Other researchers have observed a potential window for SAM stability, with more disordered SAMs being more prone to electrochemical damage.447,448
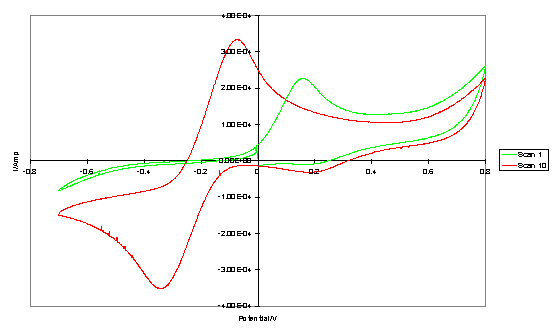
Figure 4.8
Cyclic voltammogram obtained with a gold electrode with adsorbed 223. The redox probe was Fe(CN)63-/4-. The initial scan and tenth scan are shown.
The electrochemical measurements show no differences between the monolayers of rac 184 and (R) (-) 184. Contact angles were also identical within the experimental uncertainty. Nothing can be concluded about molecular packing in the SAM from these results.
Porphyrins themselves are electroactive and it should be possible to acquire cyclic voltammograms of the porphyrin layers provided that the redox potential of the porphyrin lies within the potential window over which the layer is stable. Such studies on related porphyrins have been described in the literature,178,179,182 although due to time constraints it was not possible to pursue this line of investigation in the current work.