4.2 Reflection Adsorption Infrared Spectroscopy (RAIRS)
Attempts were made to obtain reflection-adsorption IR spectra of monolayers of 180, 181 and 260 as these compounds were expected to show informative CH and CO stretches. Using the available instrumentation satisfactory spectra of SAMS of 180 and 181 could not be obtained. The spectra displayed intense adsorptions due to the bending mode of atmospheric water426 centred at 1595 cm-1 which obscured much of the spectral region of interest. Inverted peaks were also observed, most likely arising from incorrect cancellation of the background. Additionally the purity of the samples of 180 and 181 used for this experiment was cast into doubt by the results of the XPS analysis described in section 4.1.
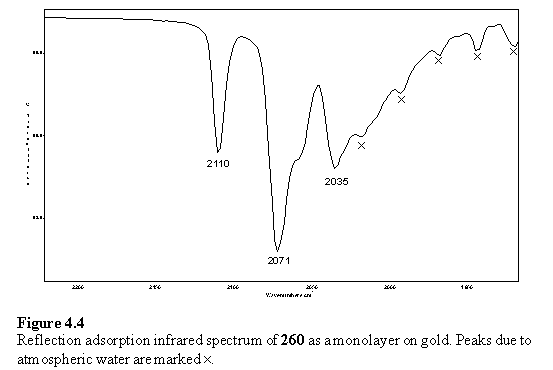
However a spectrum of 260 was obtained aided by the intense metal carbonyl absorptions which lie in a spectral region relatively unaffected by the adsorptions of atmospheric water and carbon dioxide. The spectrum (figure 4.4) is similar to the solution spectrum of 260 in CCl4 confirming the presence of the triosmium cluster on the surface. Other groups have developed methods of determining the orientation of functional groups427 and molecules428-430 in monolayers by analysis of the intensity of the IR bands observed in the monolayer spectrum compared with the corresponding bands in a solid state spectrum. This requires detailed knowledge of the direction of the transition dipole moment of each vibration, and has not been attempted for 260.
The difficulty in obtaining good quality IR spectra of monolayers can be attributed to the instrumentation, which was far from ideal for this type of sample. The angle of incidence of the IR beam to the sample was 83° which is less than the 88° required to obtain maximum surface sensitivity.431 The instrument was purged with supposedly dry air to try to eliminate interference from water vapour, although with little effect. Exchange of the dry air for nitrogen passed through a CaCl2 and KOH packed column did not improve the situation. An additional drawback to the use of ‘clean’ gold for acquisition of the background spectrum is that the gold does not remain clean once it is exposed to the atmosphere and hence does not provide a true background spectrum. Only the component of light polarized perpendicular to the metal surface can excite vibrations of the molecules of the monolayer431 so a spectrum acquired with radiation polarized parallel to the sample may be used as a background as this does not include adsorption from the monolayer. Scans acquired with polarization perpendicular and parallel to the surface may be ratioed to obtain a spectrum of the monolayer alone. With access to a polarizer this can be carried out manually, but with suitable equipment the process is rapid and automated and the technique is known as polarization modulation reflection adsorption infrared spectroscopy.432,433 Unfortunately there was no opportunity to use this technique during the course of the work presented herein.