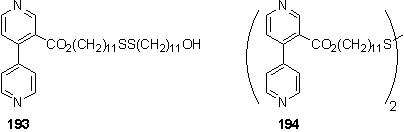3.2 Bidentate ligands
A thiol or disulfide functionalized derivative of 4,4'-bipyridine would open the door to the construction of multiporphyrin architectures, of the type described in section 2.4, on self assembled monolayer functionalized surfaces. Therefore the synthesis of such compounds was studied, making use of the wealth of bipyridine chemistry described in the literature. The chemistry is often complicated by poor solubility of these aromatic heterocycles in organic solvents and the tendency to dissolve in aqueous media as salts or zwitterions.
3.2.1 From 4,4'-bipyridine-3,3'-carboxylic acid.
4,4'-Bipyridine-3,3'-dicarboxylic acid, 188, was prepared according to the literature (scheme 3.9).361-363 The solid state structure of the acid has not previously been reported, but could be expected to show extensive hydrogen bonding due to the functional groups present. This expectation was realized in crystals grown from aqueous solution which display a cyclic hydrogen bond network between 188, which exists as a zwitterion, and water molecules (figure 3.5). The molecules are linked into infinite zig-zag chains in which the bipyridyl units are not planar but twisted by a torsion angle of 52.5°. The bipyridinium proton is hydrogen bonded to the nitrogen atom of a neighbouring pyridyl group in a linear manner, thus making another chain along the bipyridine axis with adjacent chains running in the opposite direction (figure 3.6).
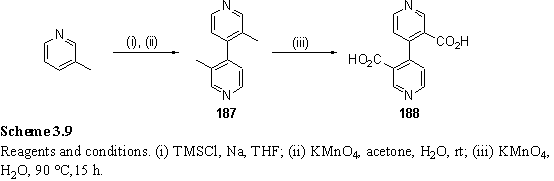
Figure 3.5
Crystal structure of 188 showing hydrogen bonding to water molecules.
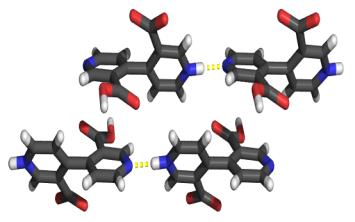
Figure 3.6
Crystal structure of 188, showing hydrogen bonds along the axis of the bipyridine.
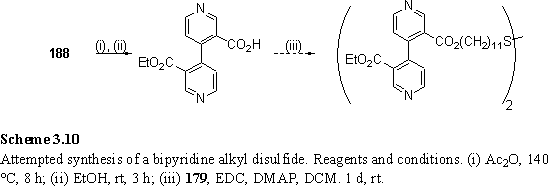
188 was treated with Ac2O according to the procedure of Rebek,363 to form a cyclic anhydride which was reacted with EtOH to form a monoethyl ester. Both of these compounds were used without purification. The material obtained was treated with 179 and EDC with DMAP to esterify the remaining carboxylic acid (scheme 3.10). Although ES MS of the products showed a significant peak at m/z 915, correct for the desired compound, purification by preparative plate chromatography yielded insufficient material for a full characterization.
3.2.2 From 4,4'-bipyridine-3-carboxylic acid
The synthesis of 4,4'-bipyridine-3-carboxylic acid, 192, has been described in the Russian chemical literature,364 but it was decided to synthesize this compound by a more modern approach making use of a Stille coupling365,366 to form the C-C bond between the aryl groups (scheme 3.11).
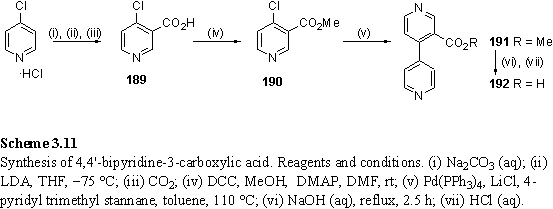
3-carboxy-4-chloropyridine, 189, was prepared by the literature route367,368 and esterified to methyl ester 190. The ester acts as a protecting group for the following step and also improves solubility and ease of purification. 190 was stored at -20 °C and used within several days of preparation as 4-halo pyridines are unstable with respect to polymerization.369 190 was coupled with 4-pyridyl trimethyl stannane to afford 191 which proved problematic to obtain pure by chromatography and recrystallization. Basic hydrolysis of the methyl ester afforded acid 192. This was esterified with 179 using the EDC/DMAP method with DCM as solvent. However a mixture of mono- and di-esterified products 193 and 194 was isolated, along with unreacted 179. The lack of reactivity may be caused by poor solubility of 192. Inclusion of DMF as a cosolvent was detrimental to the yield and gave a lower proportion of 194 relative to 193.