2.6 Self assembled porphyrin dendrimers
The ability of the Sn(IV) porphyrin to coordinate two carboxylates enables it to act as a core for the assembly of carboxylic acid functionalized dendritic wedges to form a dendrimer. This use of the tin porphyrin in conjunction with non porphyrinic dendrimer wedges has already been explored.299 Darling developed porphyrin trimer based wedges terminally functionalized with one or three pyridyl groups which coordinated Ru(II) porphyrin.300,301 Photolytic removal of the carbon monoxide ligand of the Ru porphyrin permitted two monopyridyl wedges to coordinate to a single Ru porphyrin core (section 1.6.4). However this left no free coordination sites for binding of additional porphyrins at the periphery of the dendrimer. Polymeric products could arise from Ru(CO) photolysis in the presence of the multidentate wedge.
In the following sections the use of the Rh/Sn porphyrin combination for assembly of porphyrin dendrimers will be presented.
2.6.1 Design and synthesis of wedges
For dendrimer assembly a porphyrin branching unit was required with a single carboxylic acid group for convergent binding to the Sn(IV) porphyrin core and multiple pyridyl groups for divergent coordination to Rh(III) porphyrins. The design and synthesis of such a unit followed the method of Darling,301 but replacing a single pyridyl group with an aryl carboxylic acid. Porphyrins 154 and 156 (scheme 2.9) were prepared by statistical condensations of deprotected 116 with the appropriate aldehydes using the standard procedure. The carboxylic acid group was protected as a methyl ester in 156 to improve solubility and chromatographic purification. After deprotection of the acetylene group of 154 to afford 155, this was coupled using Lindsey’s palladium catalysed conditions302 with 156 to afford the free-base porphyrin wedge 158. This synthesis permits the introduction of a metal specifically into the inner or outer porphyrin of the wedge by metallation prior to the coupling. Thus Ni(II) metallation of 156 to 157 followed by coupling to 155 yielded the mixed Ni(II) and free-base porphyrin wedge 160. Confirmation of the success of the coupling reaction was provided by the 1H NMR spectra of the wedges which displayed two meso peaks in a 2:1 ratio corresponding to the inner and outer porphyrins. Also the expected molecular ions were observed in the MALDI TOF mass spectra. The methyl ester resonance of 158 and 160 appeared as a singlet at 4.15 and 4.11 ppm respectively. 158 displayed three pyrrolic proton peaks due to slow exchange of the inner porphyrin NH protons.
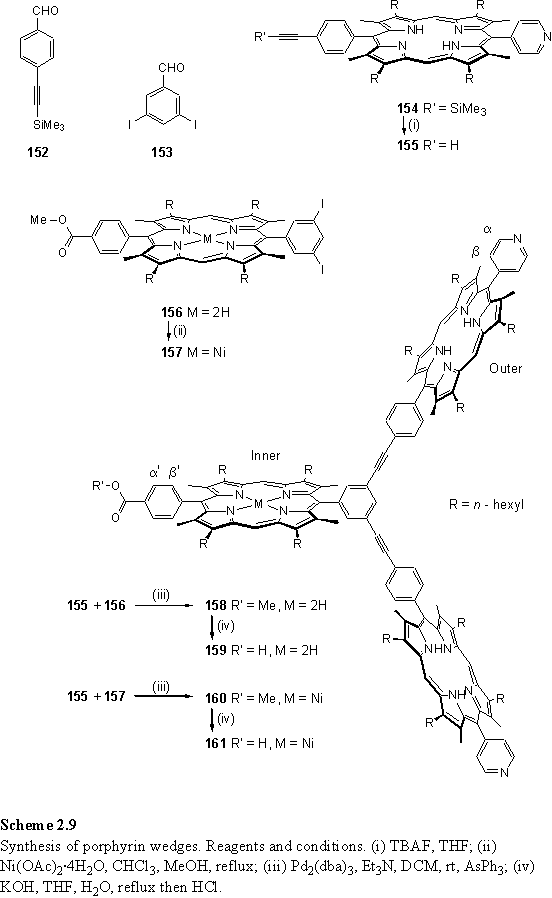
The carboxylic acid groups were deprotected using the same conditions used for 147, namely potassium hydroxide in refluxing wet THF. The 1H NMR of the acid functionalized wedges 159 and 161 were essentially the same as their methyl ester protected counterparts, but lacking the methyl ester singlet.
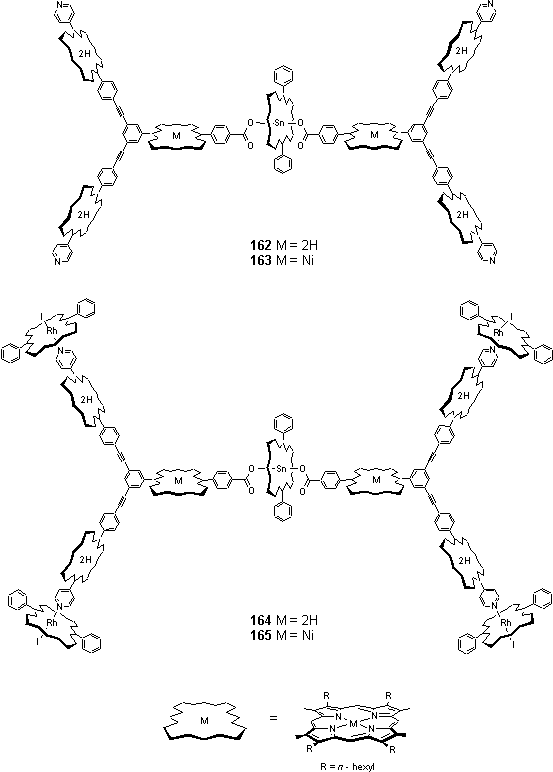
2.6.2 Assembly of Sn/fb/Rh porphyrin dendrimer
The assembly of dendrimers was carried out as a two stage titration, monitored by NMR spectroscopy. This enables the observation and characterization of intermediates in the assembly process.
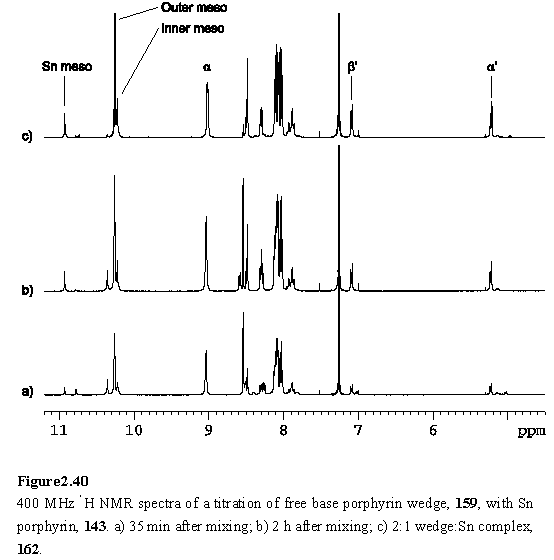
Tin porphyrin 143 was mixed with 3 equivalents of wedge 159 in CDCl3 and the 1H NMR spectrum (figure 2.40 a) recorded 35 minutes after mixing. The spectrum shows the peaks assigned to the 2:1 wedge:tin porphyrin complex 162 and an intermediate species postulated to be the 1:1 complex, along with the resonances of unreacted 159. After two hours the peaks due to the intermediate had diminished in intensity as this reacted with 159 to yield 162. Aliquots of 143 solution were titrated in over a period of two days, with NMR monitoring, until the predominant species in solution was 162. The resonances of the bound aryl carboxylate of the inner porphyrin appear clearly in the spectrum as a pair of doublets at 7.09 and 5.22 ppm (figure 2.40 c).
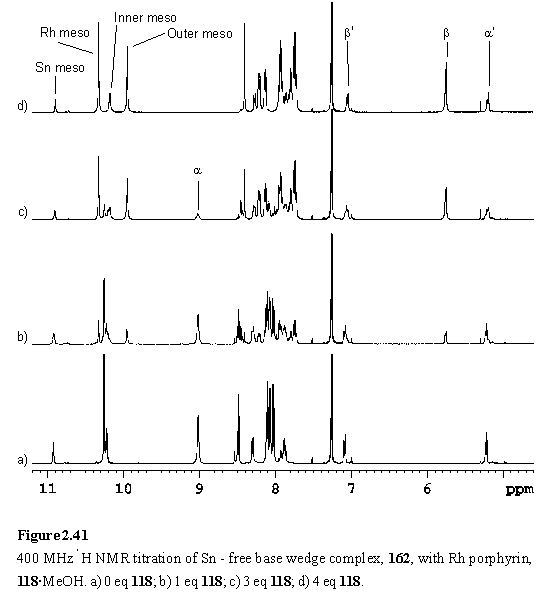
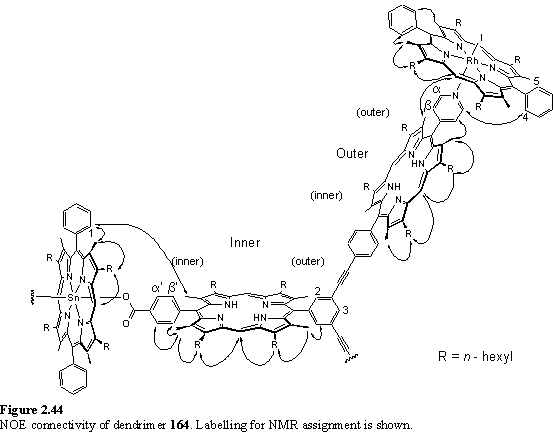
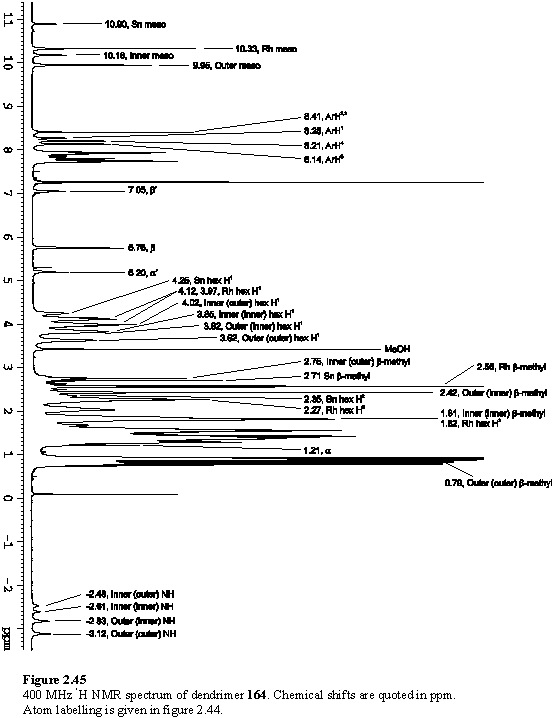
Into the solution of 162 was titrated 118·MeOH. Addition of less than four equivalents of the Rh porphyrin yielded complex NMR spectra with multiple meso resonances (figure 2.41 b, c). This arises from the existence of species in which zero to four of the peripheral pyridyl groups of 162 are coordinated to 118. There are a total of six such species, including 162 itself, so spectral assignment has not been possible. The intensity of the pyridyl Ha resonance at ~9.0 ppm decreased and a new doublet appeared at 5.76 ppm assigned to Hb of pyridyl groups coordinated to 118. After addition of four equivalents of 118 the spectrum (figure 2.41 d) simplified considerably as the intermediates with non-coordinated pyridyl groups were no longer present, leaving 164 as the major species in the solution. COSY and NOESY spectra were obtained to assist in assignment of the resonances of 164.
The NOESY spectrum (figures 2.42, 2.43) is especially useful in this respect, as the substituents at the porphyrin periphery are spatially close to one another but there is no coupling between them.
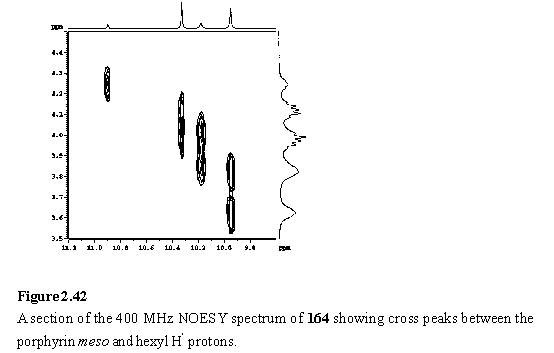
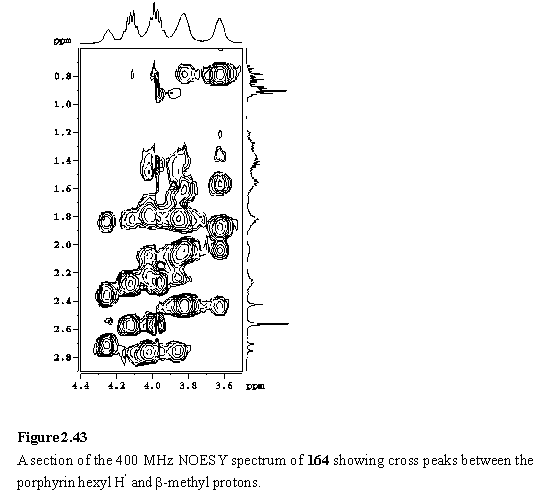
The NOE connectivity (figure 2.44) can be followed from the meso peaks, to the hexyl chains and from here to the b-methyls. The b-methyls in turn show NOESY cross peaks to the adjacent aryl protons. NOEs to the pyridyl and aryl carboxylate protons enable the inequivalent b-methyl and hexyl groups of the inner and outer free-base porphyrins to be unambiguously assigned. The inner(inner) and outer(outer) b-methyls of the wedge are closest to the Sn and Rh porphyrins respectively, and as a consequence are shielded, and were located amongst the hexyl resonances at 1.81 and 0.79 ppm respectively. The pyridyl Ha resonance was identified as a doublet at 1.21 ppm from its cross peak with Hb in the COSY spectrum. The hexyl resonances overlap extensively, but were assigned (table 2.1) from a TOCSY spectrum. Additional support for the proposed structure of the array comes from the observation of a weak NOE between the outer(outer) b-methyl group and the Rh meso proton confirming the close spatial proximity of the two porphyrins enforced by Rh-pyridyl coordination. A weak NOE between a doublet at 8.21 ppm, assigned to one of the Rh porphyrin aryl groups, and the pyridyl Ha enables this doublet to be identified as the aryl H4 (for numbering see figure 2.44) which faces towards the pyridyl ligand. The Rh aryl H5 is assigned to a doublet at 8.14 ppm which displays an NOE to the Rh b-methyl. Another weak NOE is observed between the doublet at 8.28 ppm, assigned to the Sn porphyrin aryl group and the inner(inner) b-methyl.
|
|
H1 |
H2 |
H3 |
H4 |
H5 |
H6 |
|
Sn |
4.25 |
2.35 |
1.84 |
1.49 |
1.33 |
0.75 |
|
Inner (inner) |
3.85 |
2.08 |
1.68 |
1.44 |
1.35 |
0.90 |
|
Inner (outer) |
4.02 |
2.22 |
1.77 |
1.50 |
1.39a |
0.92 |
|
Outer (inner) |
3.82 |
2.03 |
1.61 |
1.37 |
1.27 |
0.82 |
|
Outer (outer) |
3.62 |
1.88 |
1.56 |
1.37 |
1.31 |
0.87 |
|
Rh |
4.12 3.97 |
2.27 |
1.83 |
1.56 |
1.42 |
0.90 |
Table 2.1
Chemical shifts (ppm) of the hexyl groups of 164. aThis chemical shift is approximate due to peak overlap. The hexyl numbering scheme is given in figure 2.3.
As was observed with 150, the exchange of the pyrrolic protons is slow on the chemical shift time-scale and this results in two NH resonances for each of the inner and outer free-base porphyrins. The more upfield shifts of the outer NH protons reflect both a feature of the wedge itself, and greater additional shielding of the outer NH protons by the Rh porphyrin than the inner protons are shielded by the more distant Sn porphyrin. The full 1H NMR spectrum of 164, with assignments, is given in figure 2.45.
2.6.3 Sn/Ni/fb/Rh dendrimer
The assembly of the dendrimer, 165, was accomplished from the components 161, 143 and 118 by a procedure analogous to that used to prepare 164. The Ni porphyrin meso resonance of the intermediate 163, appears well upfield of the free-base meso resonance (figure 2.46 a). This is an inherent property of the Ni porphyrin, with the meso resonance of the Ni porphyrin unit of 161 appearing at 9.54 ppm, whilst the corresponding free-base porphyrin peak of 159 is observed at 10.35 ppm.
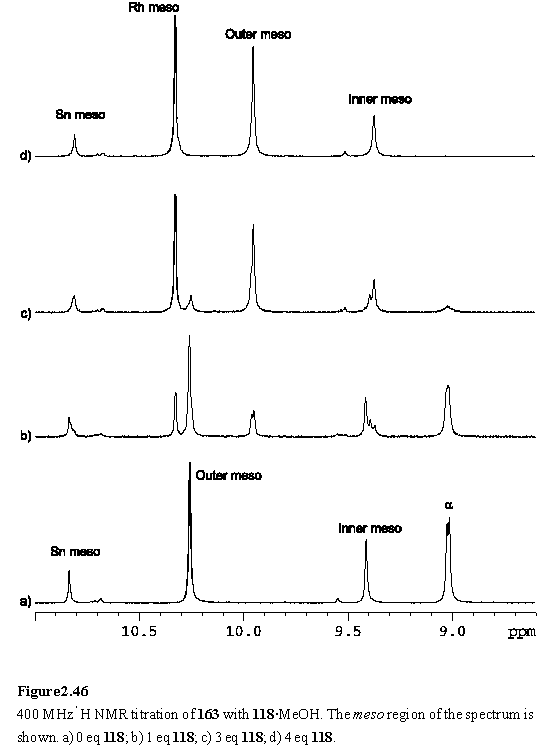
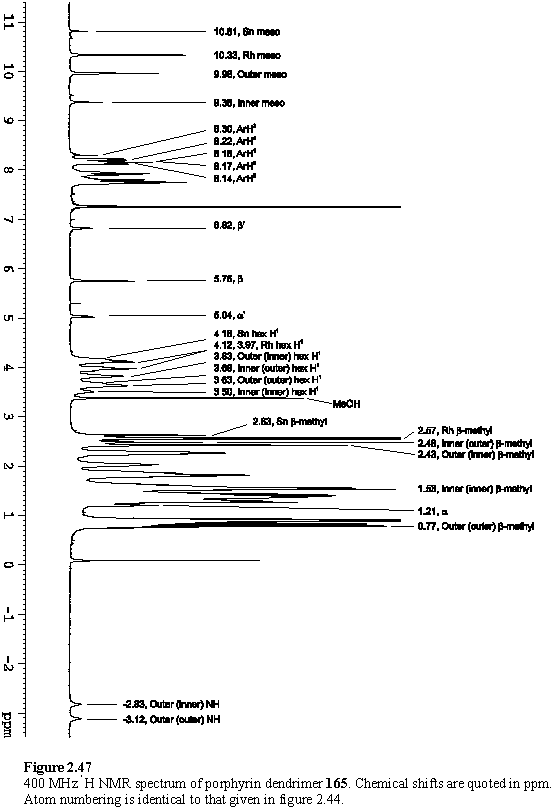
As was observed for 162, addition of less than four equivalents of 118 yielded complex NMR spectra (figure 2.46 b, c), which simplified considerably once the addition of 4 eq was complete. The COSY and NOESY spectra were interpreted as discussed in the previous section for 164, and enabled assignment of most of the resonances of 165 (figure 2.47, table 2.2).
|
|
H1 |
H2 |
H3 |
H4 |
H5 |
H6 |
|
Sn |
4.18 |
2.28 |
1.79 |
1.46 |
1.31 |
0.76 |
|
Inner (inner) |
3.51 |
1.88 |
1.54 |
1.38 |
1.32 |
0.88 |
|
Inner (outer) |
3.68 |
2.03 |
1.65 |
1.45 |
1.37a |
0.91 |
|
Outer (inner) |
3.83 |
2.04 |
1.61 |
1.37 |
1.27 |
0.83 |
|
Outer (outer) |
3.63 |
1.88 |
1.56 |
1.38 |
1.30 |
0.88 |
|
Rh |
4.12 3.98 |
2.27 |
1.82 |
1.56 |
1.42 |
0.90 |
Table 2.2
Chemical shifts (ppm) of the hexyl groups of 165. aThis chemical shift is approximate due to peak overlap. The hexyl numbering scheme is given in figure 2.3.
The MALDI TOF mass spectrum of a mixture of 143, 161 and 118 did not show a molecular ion peak for 165. However a peak at m/z 3686 was observed which can be assigned to a 1:1 complex of 143 and 161 with loss of the other hydroxyl group from 143. However it cannot be concluded that this peak is due to the specific association of 143 and 161 through axial porphyrin ligation as the mass spectrum does not provide structural information. The only other significant peak at a mass greater than monomeric porphyrins was observed at m/z 1928 suggesting a dimeric aggregate of porphyrins.