1.7 Self assembled monolayers of porphyrins
A number of groups have investigated self assembled monolayers of porphyrins for applications ranging from electrocatalysis to sensing and photosynthetic models. The Langmuir-Blodgett technique offers another means of preparation of monolayer films on solid supports, although layers prepared by this method will not be discussed here.
1.7.1 Porphyrin peripheral binding to surfaces
The most common approach to porphyrin self assembled monolayers has been the adsorption to gold surfaces of porphyrins with peripheral thiol or disulfide groups. However alternatives have been reported, including quaternerization of pyridyl porphyrins with benzyl chloride functionalized silica surfaces,167 coupling of a pentafluorophenol active ester porphyrin with aminopropylsilylated glass168 and addition of the alkene moiety of hematoporphyrins to surfaces exposing a reactive thiol group.169,170
The syntheses of a variety of sulfur functionalized porphyrins for self assembly on gold have been described in detail. Yuan and Woo chose to use an amide group to link a trityl protected alkane thiol to a porphyrin.171 Porphyrin metallation with Co(II) or Zn(II) was carried out on the protected compound, followed by trityl group removal by treatment with Hg(OAc)2 then H2S, to afford thiols 103 and 104. In a recent paper Lindsey and coworkers investigated the preparation of porphyrin thiol derivatives with a range of thiol protecting groups to assess the stability of these groups to the conditions required for porphyrin synthesis (TFA + BF3·Et2O) and Pd coupling reactions for preparation of covalently linked multiporphyrin arrays.172 The thioacetyl group was found to be compatible with both conditions, and additionally the protected porphyrins were found to adsorb to gold electrodes possibly by in situ thioacetate cleavage, thus obviating the need to handle air sensitive thiols. Similarly Jagessar and Tour have prepared porphyrin ‘alligator clips’ such as 105 with a view to assembly between gold electrodes for the construction of molecularly sized electronic components.173
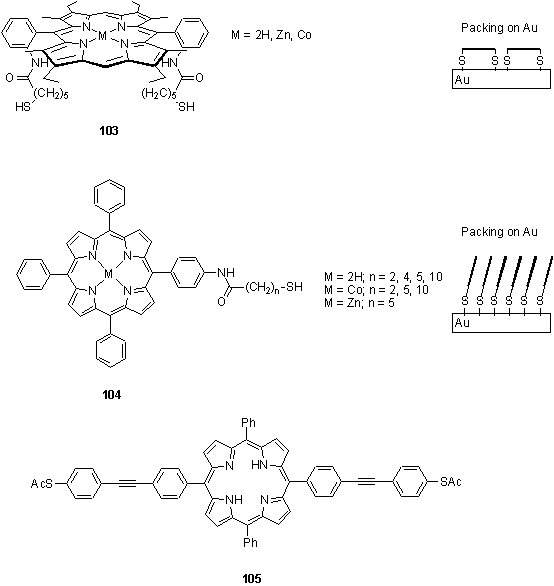
The structure of monolayers of 103 and 104 on gold surfaces was investigated by XPS and UV/visible spectroscopies.174 XPS indicated that for 104 (M = Co, n = 5) all sulfur was bound to the gold surface as thiolate, although for 103 (M = Co) the XPS results could not rule out traces of polysulfide. The UV/visible spectrum of the mono thiol compound showed a split Soret band which was interpreted to be due to exciton coupling between cofacial tilted porphyrins. The spectrum of dithiol 103 (M = Co, n = 5) displayed a red shifted Soret peak which was attributed to exciton coupling between edge to edge oriented porphyrins implying the porphyrin plane lay parallel to the gold surface. Attempts to metallate monolayers of free-base 103 and 104 with Co(OAc)2 were only successful for 103, as measured by the ability of the monolayers to catalyse electrochemical oxygen reduction compared to the analogous monolayers prepared with premetallated porphyrin. This also supports the hypothesis that 103 packs with the porphyrin parallel to the gold, with the metal site exposed, whereas 104 stacks such that the core of the porphyrin is inaccessible.
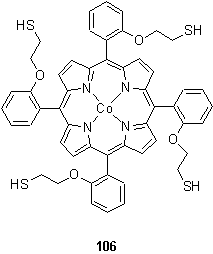
Murray et al. examined electrocatalytic oxygen reduction by monolayers of 106 on gold, and concluded from XPS measurements of the S 2p binding energy that approximately three quarters of the thiol groups were bound to the gold surface which necessitates a parallel arrangement of the porphyrin and gold.175,176 These workers proposed that oxidation of Co(II) to Co(III) occurred on monolayer formation, on the basis of the UV spectra of monolayer electrodes as a function of potential. 14 nm of the 24 nm red shift of the Soret band observed on monolayer formation was attributed to this oxidation process, and the remainder to exciton coupling between edge to edge packed porphyrins.
Similar conclusions, based on UV spectra, about the role of the number of thiol groups on the orientation of the porphyrin moiety were reached by the group of Shimazu.177 A recent study of the effect of the alkyl chain length of monolayers of 107 on gold revealed an oscillation in properties (absorbance, fluorescence lifetime) between compounds with an odd and even number of methylene groups which was attributed to an alternation in the tilt angle of the porphyrin with respect to the surface.178 Others have studied similar monolayers by XPS, electrochemistry179 and surface plasmon enhanced fluorescence spectroscopy.180,181
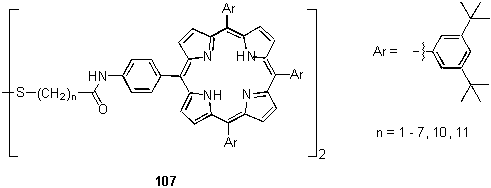
More elaborate monolayers of porphyrins and electron donors/acceptors have been prepared on gold electrodes with a view to mimicking photosynthetic light harvesting and vectorial electron transfer processes. Such studies include an investigation of in-plane energy transfer between 107 (n = 11) and a disulfide derivatized pyrene in a mixed monolayer,182 and electron transfer in monolayers of porphyrin - quinone,183,184 ferrocene185,186,187 and fullerene186,187,188 dyads and triads. The photophysics and spectroelectrochemistry of these systems is outside the scope of this review.
McLendon used monolayers of thiol functionalized heme groups to reconstitute apomyoglobin.189 The binding of myoglobin to the monolayer was monitored by the electrochemical response of the Fe(III) heme group and the fluorescence response of a Zn(II) derivative. Intact myoglobin with the heme prosthetic group already bound did not interact with these monolayers, confirming a specific interaction of the apoprotein with the monolayer. The authors proposed that this could be a general method of forming monolayers of proteins which bind a prosthetic group.
The application of metalloporphyrin monolayers as sensors for volatile compounds was investigated using the quartz crystal microbalance technique.190 The frequency change of quartz crystal oscillators with gold electrodes functionalized with metalloporphyrins 108 - 112 was measured as a function of the concentration of various organic vapours. It was found that the tetrathiol porphyrin 112 exhibited a higher response than monothiol 111 to ethanol and triethylamine, but a lower response to ethylene diamine. This was attributed to greater accessibility of the metal centre of 112 compared to 111 in which the porphyrins may stack thus inhibiting axial ligand binding. However ethylene diamine may chelate (figure 1.15) between two porphyrins of 111 but is too short to span between the metal centres of 112. Chelation of bidentate ligands between porphyrins immobilized on a glass surface has also been reported.168 In this case it was found that DABCO was less able to bridge between porphyrins than 4,4'-bipyridine and 1,3-di(4-pyridyl)propane most likely due to the short length of the former ligand.
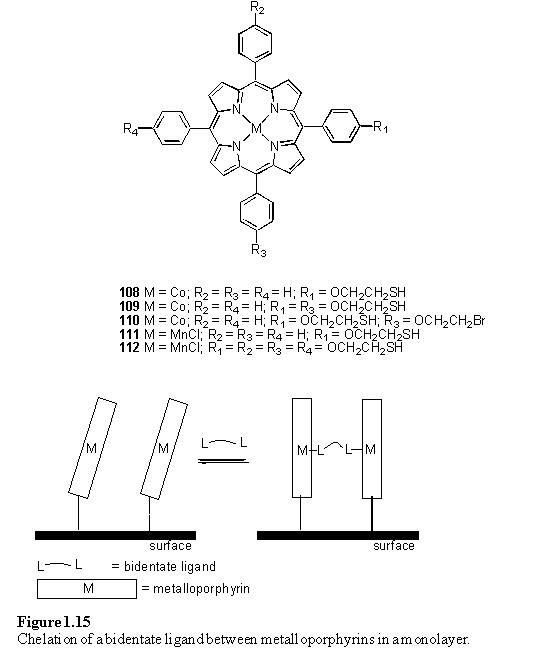
However caution must be exercised when interpreting the results of such experiments in terms of axial coordination of ligands to metalloporphyrin monolayers; the authors of reference 190 also remarked that their porphyrin functionalized microbalances were sensitive to benzene and hydrocarbons despite the fact that these compounds do not have donor atoms for porphyrin coordination. In another paper from the same group Co(II) and free-base porphyrin monolayers were shown to be equally effective sensors for ethanol, acetic acid and triethylamine, even though the free-base porphyrin cannot coordinate an axial ligand.191
1.7.2 Axial coordination of metalloporphyrins to monolayers
An alternative approach to the preparation of porphyrin containing monolayers is the axial coordination of porphyrin to a preformed monolayer presenting a porphyrin binding ligand.
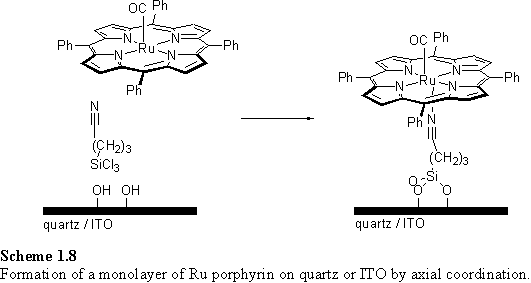
Reaction of a mixture of cyano alkyl chlorosilane and (TPP)Ru(II)CO with a quartz or ITO surface resulted in immobilization of the porphyrin on the surface (scheme 1.8).192 The UV absorbance of the samples indicated a dense packing of the porphyrins on the surface, using the crystallographically determined geometry of the porphyrin to estimate its molecular area.
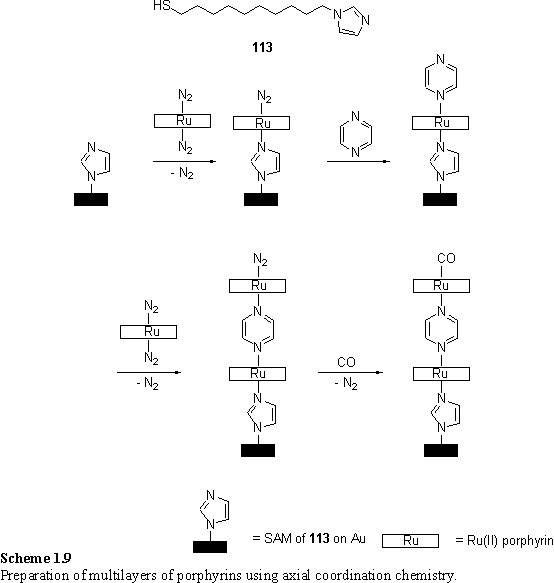
Collman and coworkers outlined the preparation of multilayer porphyrin structures using axial ligation of Ru(II) and Os(II) porphyrins.193 A mixed SAM of imidazole thiol 113 and nonanethiol was immersed in a solution of a Ru(II) porphyrin with labile ligands, such as acetonitrile or dinitrogen. Displacement of one of these ligands by the imidazole terminated monolayer anchored the porphyrin to the surface. A remaining weakly coordinated dinitrogen ligand could be removed by dipping the substrate into a solution of the bidentate ligand pyrazine. Further exposure to the porphyrin solution deposited another layer of axially coordinated porphyrin. This process could be repeated until finally the layer was ‘capped’ by placing under a CO atmosphere (scheme 1.9). Evidence for the formation of the assemblies by specific axial coordination was provided by reflection adsorption IR spectra of a layer of (tetramesitylporphyrin)Ru(II)CO which displayed a shifted CO stretch relative to the bulk compound, consistent with a trans effect from coordinated imidazole. No porphyrin binding to a pure nonanethiol SAM was observed and porphyrins with strongly bound axial ligands did not adsorb to the imidazole terminated SAMs. STM images of the surfaces showed disc-like features of a size consistent with the known dimensions of the porphyrin. Multilayer formation was evidenced by ellipsometric thickness measurements and the relative intensities of the C 1s, Ru 3d and Au 4f peaks in the X-ray photoelectron spectrum.
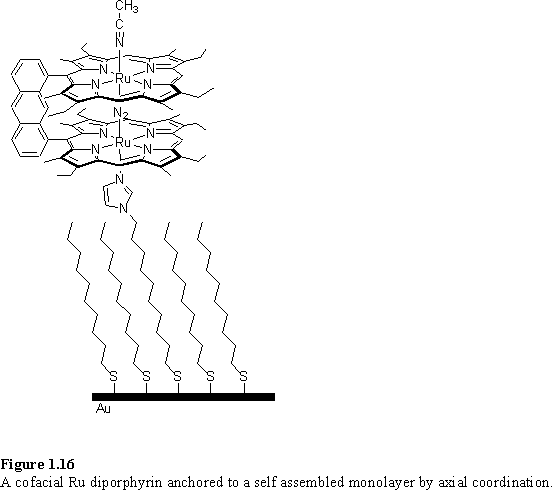
This same chemistry was used to anchor a cofacial diporphyrin to a surface (figure 1.16).194 The monolayer catalysed the electrochemical reduction of dioxygen, and experiments demonstrated that the mechanism most likely involved loss of the dinitrogen ligand and binding of dioxygen between the porphyrins.
Other recent reports have described the interaction of (TPP)Co with a pyridine thiol monolayer on gold195 and binding of protoporphyrin IX Zn to aminopropylsilylated glass surfaces.196 The latter report claimed the monolayers to be stable to HCl treatment and immersion in ethanolic imidazole solution, a result which seems surprising given the lability of zinc porphyrins and the tendency to demetallate under acid conditions.
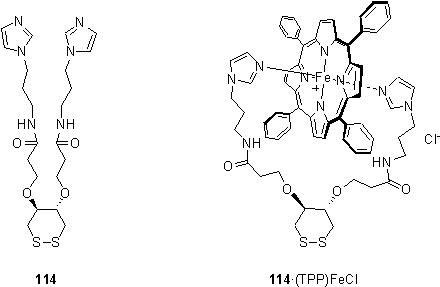
In order to orient a porphyrin perpendicular to a gold surface using coordination chemistry Rubenstein and Shanzer synthesized a chelating ligand 114 capable of spanning the faces of a six coordinate porphyrin.197 The ligand was shown to bind to (TPP)FeCl in solution, and a monolayer of this preformed complex was prepared. For comparison a monolayer of 114 was prepared and subsequently exposed to (TPP)FeCl. This was found to lead to an increase in the ellipsometric thickness of the monolayer. Monolayers formed by either procedure displayed the expected XPS peaks of Fe. However UV spectroscopy and cyclic voltammetry indicated a larger quantity of porphyrin in the monolayer prepared from the preformed complex 114·(TPP)FeCl, than that assembled stepwise. This would imply that the conformation that 114 adopts in a monolayer may be unfavourable for binding of the porphyrin. A monolayer of 114·(TPP)FeCl on CdSe single crystals was found to reversibly bind dioxygen, although the mechanism of binding was not clear.198