1.1 Self assembled systems with two building blocks
The simplest systems are composed of two building blocks, comprising a single type of multidentate ligand and a single type of metal ion to which it binds. Lehn et al. have reported the preparation of a variety of grid shaped complexes from perpendicular arrays of polypyridyl ligands. Transition metals occupy the intersections of the grid lines formed by the ligands. These were prepared with a view to the creation of individually addressable arrays of redox active sites for use as an information storage medium.
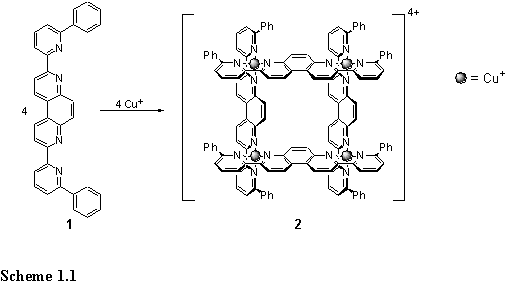
A grid, 2, consisting of four Cu(I) ions and four ligands, 1, was prepared by mixing the ligand and Cu(MeCN)4PF6 in a 1:1 ratio in nitromethane (scheme 1.1).3 The preference of Cu(I) for tetrahedral coordination requires that two ligands are brought together in an orthogonal arrangement at each metal. The grid structure fills the coordination sphere of each metal, and leaves no free ligand sites for further metal coordination. The symmetric nature of the product in solution was revealed by the 13C NMR spectrum which displayed only 15 peaks. The spacing between the metal centres was deliberately large enough to create a box like structure with sufficient internal volume to include small molecules. Indeed crystallography revealed inclusion of four benzene and two nitromethane molecules in the grooves of the grid in the solid state. Importantly, the phenyl substituents were found to be essential for assembly of the grid in preference to insoluble and presumably polymeric material. Steric clash between these substituents is likely to disfavour polymeric structures.
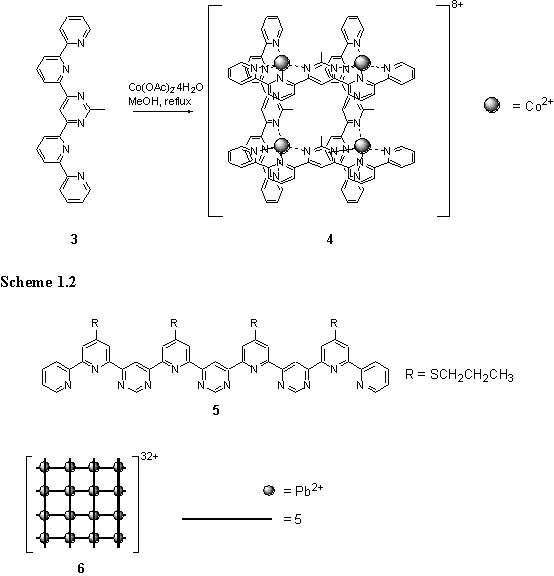
The same strategy was applied to assemble a grid, 4, from octahedral Co(II) and a ligand, 3, based on tridentate terpyridine (scheme 1.2).4 Although paramagnetism precluded NMR spectroscopy, the presence of the grid structure in the solution and solid state was attested to by elemental analysis, electrospray mass spectrometry and crystallography. Once again, steric clash between ligands was invoked to explain the absence of polymeric products.
By extending the number of chelating coordination sites on the ligand, 5, larger square grids, up to a 4 ´ 4 array, were assembled around Pb(II) ions.5 The 1H NMR spectrum indicated clean formation of the grid, 6, with two ligand environments consistent with the proposed structure. Additional support for the structure came from the 207Pb NMR spectrum which displayed three peaks in a 1:2:1 intensity ratio.
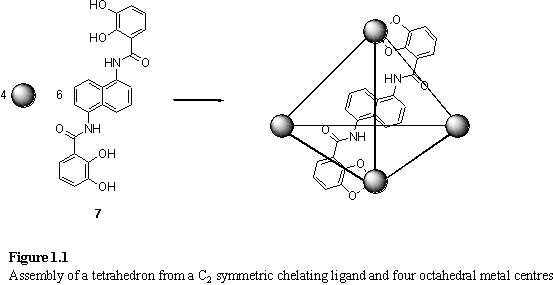
 Raymond et al. have
designed a range of tetrahedral assemblies with metal ions as vertices by
careful consideration of the preferred geometry of the metal ion and the
symmetry of the vertices, edges and faces of the tetrahedron.6 If a
metal with a preferred idealized octahedral geometry is used as the vertex of a
tetrahedron then the C3 symmetry of the vertex requires three
bidentate ligands (figure 1.1). If the ligands are to occupy the edges of the
tetrahedron, this requires they possess C2 symmetry. A chelating
ligand, 7, which satisfies the C2
requirement was designed and after stirring with Ga(acac)3, KOH, and
Et4NCl in MeOH a precipitate was observed and formulated as K5[Et4N]7[Ga476]·8H2O.7
After dissolution in D2O, the 1H NMR spectrum indicated
quantitative formation of a tetrahedral species, in which an Et4N+
cation was encapsulated, as evidenced by upfield shifted resonances at -0.68
and -1.58
ppm. The X-ray structure of the Fe(III) analogue was obtained, and confirmed
the inclusion of the Et4N+ within the tetrahedron. The
naphthalene spacer unit of the ligand avoids formation of a triple helicate by
offsetting the catechol groups when they are in a cis conformation with respect to each other. Otherwise, on entropic
grounds, the formation of a triple helicate would be preferred.
Raymond et al. have
designed a range of tetrahedral assemblies with metal ions as vertices by
careful consideration of the preferred geometry of the metal ion and the
symmetry of the vertices, edges and faces of the tetrahedron.6 If a
metal with a preferred idealized octahedral geometry is used as the vertex of a
tetrahedron then the C3 symmetry of the vertex requires three
bidentate ligands (figure 1.1). If the ligands are to occupy the edges of the
tetrahedron, this requires they possess C2 symmetry. A chelating
ligand, 7, which satisfies the C2
requirement was designed and after stirring with Ga(acac)3, KOH, and
Et4NCl in MeOH a precipitate was observed and formulated as K5[Et4N]7[Ga476]·8H2O.7
After dissolution in D2O, the 1H NMR spectrum indicated
quantitative formation of a tetrahedral species, in which an Et4N+
cation was encapsulated, as evidenced by upfield shifted resonances at -0.68
and -1.58
ppm. The X-ray structure of the Fe(III) analogue was obtained, and confirmed
the inclusion of the Et4N+ within the tetrahedron. The
naphthalene spacer unit of the ligand avoids formation of a triple helicate by
offsetting the catechol groups when they are in a cis conformation with respect to each other. Otherwise, on entropic
grounds, the formation of a triple helicate would be preferred.
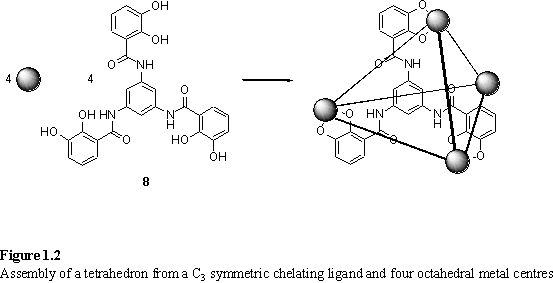
If a ligand is to occupy the faces of the tetrahedron then C3 symmetry is required (figure 1.2). The ligand 8 was designed for this purpose.8 On initial treatment with Ti(OnBu)4 and Et3N in MeOH a precipitate appeared which was ascribed to a mixture of polymers, formed as the kinetic products. On refluxing this in DMF with Et3N for 12 h a single product, [Ti484]8- was observed by 1H NMR and ES MS consistent with a tetrahedral species. An important feature is that the kinetic intermediates could be converted, by ligand exchange, into the desired thermodynamic product. Crystallography confirmed the nature of the product in the solid state.
Fujita has described the assembly of capsules, 9 and 10, from tripyridyl triazine (TPT) and Pd(II) or Pt(II).9 Each metal centre forms a vertex of the capsule at which the ethylene diamine ligand forces the two TPT ligands to adopt a cis geometry. The Pd capsule has a diameter of ~2 nm and was found to complex large neutral molecules such as adamantane10 which was extracted from a hexane solution into an aqueous solution of 9. The progress of the encapsulation could be observed by 1H NMR which indicated each molecule of 9 bound four molecules of adamantane. The adamantane resonances were shifted upfield by up to 2.2 ppm due to the chemical shift anisotropy of the aromatic groups comprising the walls of the capsule.
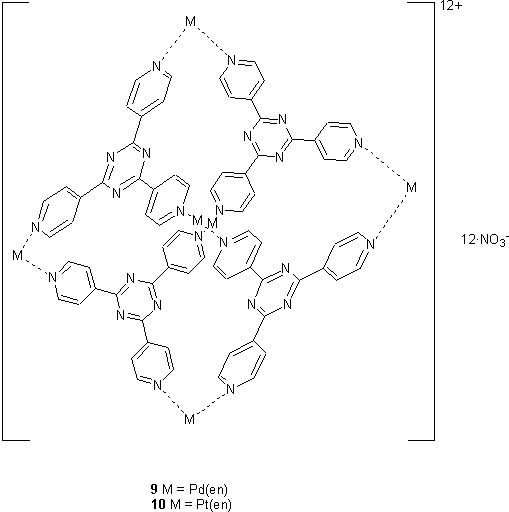
On mixing Pt(en)(ONO2)2 and TPT in D2O initially a mixture of products was observed by 1H NMR. On heating the sample to 100 °C for 24 h, the spectrum simplified slightly implying some conversion to the capsule 10. However on addition of sodium adamantane carboxylate, 11, and heating for a further 24 h at 100 °C, 10 was observed as almost the exclusive product. Four molecules of 11 were included inside the capsule. It thus appears that 11 acts as a template for the capsule to which it binds. 11 could be removed from the capsule by treatment with nitric acid then extraction with chloroform. Due to the inertness of the Pt(II) centres at room temperature the structure of the capsule is frozen, and no reversion to other oligomeric species was observed during or after removal of the guest.11